42 the ideal gas law worksheet
Ideal gas law practice worksheet with answers Problem (PageIndex {1}) Sometimes leaving a bicycle under the sun on a hot day causes a burst. Why? Answer as a temperature of a gas increases, the pressure will also increase according to the ideal gas law. The tire volume can only expand so much earlier the rubber dà and releases the accumulation ...
View ideal gas law worksheet (1).pdf from FS1 101 at New Orleans Military Maritime Academy. IDEAL GAS LAW WORKSHEET SET 1: Problem #1: Determine the volume of occupied by 2.34 grams of carbon
Title: Ideal Gas Law Problems Author: Dan Keywords: ideal gas law, practice sheet Created Date: 3/5/2000 4:41:40 PM

The ideal gas law worksheet
2.2 If the gas's volume is changed to ½ V and its temperature to 2T, what will the gas's pressure be? 2.3 If the gas's volume is changed to 4 V and its pressure to ¼ V, what will its temperature be? Question 3 A 2 cm3 gas bubble rises from the bottom of the sea, where the pressure is 275 kPa and the temperature 5°C.
Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, “PerV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems: K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atm to get R =8.31 kPa*L / (K*mole) 1) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 ...
CHEMISTRY GAS LAW’S WORKSHEET 5. A sample of gas has a volume of 215 cm3 at 23.5 °C and 84.6 kPa. What volume will the gas occupy at STP? 4. 8.98 dm3 of hydrogen gas is collected at 38.8 °C. Find the volume the gas will occupy at -39.9 °C if the pressure remains constant. 3. A sample of nitrogen gas
The ideal gas law worksheet.
Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, "PerV-nRT", and the universal gas constantR = 0.0821 L*atm to solve the following problems:K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atmto get R =8.31 kPa*L / (K*mole)
Given: Ideal Gas Law = then P = n = V = T = R = What pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 L container at a . temperature of 20.(C? Oxygen gas is collected at a pressure of 123 kPa in a container which has a volume of 10.0 L.
Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, “PV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems: K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atm to get R =8.31 L*kPa / (K*mole) 1) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 liters ...
Ideal gas law practice problems worksheet.Stp is 273 k and 1 atm 101 325kpa 760torr 760mmhg. Ptotal p p p p1v1 t1 p2v2 t2 pv nrt v in l or dm3 in ideal gas law p in atm when r 0 0821l atm mol k.
The ideal gas law states that pv nrt where p is the pressure of a gas v is the volume of the gas n is the number of moles of gas present r is the ideal gas constant and t is the temperature of the gas in kelvins. Solutions to the ideal gas law practice worksheet. A sample of carbon monoxide at 57oc and under 0 67 atm of pressure takes.
The ideal gas law states that pv nrt where p is the pressure of a gas v is the volume of the gas n is the number of moles of gas present r is the ideal gas constant and t is the temperature of the gas in kelvins. Ideal gas law practice worksheet solve the following problems using the ideal gas law.
PDF. This is a single 2-page worksheet with problems utilizing the ideal gas law. Students will solve for each of the variables, and for molar mass. There are a total of 8 problems.Answer key is included.The download includes a handout master (.pdf) that includes one worksheet, and answer key.This produc. Subjects:
These ( pdf) are Ideal Gas Law problems and these ( pdf) are both Combined Gas Laws and Ideal Gas Law Problems. This worksheet ( doc) is a review of all the gas laws. Have students try this "Gas Laws Magic Square" ( doc). Do this Gas Laws crossword puzzle ( doc) or try this "Gases" ( pdf) crossword with answers.
Worksheet 7 - Ideal Gas Law I. Ideal Gas Law The findings of 19th century chemists and physicists, among them Avogadro, Gay-Lussac, Boyle and Charles, are summarized in the Ideal Gas Law: PV = nRT P = pressure V = volume n= moles of gas, R = universal gas constant T = temperature. The value of R varies with the units chosen: R = 0.08206 L atm / mol K
Collection Of Gas Law Activities Ideal Gas Law Cooperative Learning Activities Activities. Chemical Bonding Crossword Worksheet Answers Worksheets Are A Crucial Part Of Studying English Todd In 2021 Puzzles And Answers High School Science Crossword Puzzle. Ideal Gas Law Practice Problems Ideal Gas Law Chemistry Lecture Lectures Notes.
Practice Ideal Gas Law Worksheet: 1 - 4 (page 8 in packet) Gas Stoichiometry Molar Volume - 1 mol of any gas at STP has a volume of 22.4 L 1 mol = conversion factor (only to be used at STP) 22.4 L Example: What is the mass of 98.0 ml of sulfur dioxide at STP? (work together on board - copy
Ideal gas law worksheet pdf. K mol if pressure is needed in kpa then convert by multiplying by 101 3kpa 1atm to get r 8 31 kpa l k mole. The value and units of. Use the ideal gas law and the universal gas constant r 0 0821. R 8 31 kpa atm k mole 1 if i have 4 moles of a gas at a pressure of 5 6 atm and a volume of 12 liters what.
CH301 Worksheet 8—Gases (Answer Key) 1. What do we assume about ideal gases? What is the ideal gas law? Give the units for each variable. Ideal gases are infinitely small, hard spheres that do not interact with each other. They are essentially "blind" to other gas molecules and will bounce off of each other just as they would bounce of a wall.
Ideal Gas Law: Density and Molar Mass. 8. At what Celsius temperature will argon have a density of 10.3 g/L and a pressure of 6.43 atm? (31 deg. C) 9. The density of an unknown gas at 20oC and 749 mm Hg is 1.31 g/L. Calculate the molar mass of the gas. ... Gas Laws Review Worksheet ...
Diy The Ideal Gas Law Worksheet Sketch on the graph below how the volume of a gas changes as the number of moles of gas is increased. The ideal gas law states that pv nrt where p is the pressure of a gas v is the volume of the gas n is the number of moles of gas present r is the ideal gas constant and t is the temperature of the gas in kelvins.
Chemistry: The Ideal Gas Law KEY Directions: Solve each of the following problems.Show your work, including proper units, to earn full credit. 1. If 3.7 moles of propane are at a temperature of 28oC and are under 154.2 kPa of pressure, what volume does the sample occupy?
Solutions to the Ideal gas law practice worksheet: The ideal gas law states that PV=nRT, where P is the pressure of a gas, V is the volume of the gas, n is the number of moles of gas present, R is the ideal gas constant, and T is the temperature of the gas in Kelvins. Common mistakes: Make sure you T in Kelvins, rather than degrees celsius.
2. $2.00. PDF. Chemistry worksheet on the topic of the Ideal Gas Law - one of the fundamental Gas Laws. This worksheet contains an explanation of the relationship between the volume of a gas, pressure of a gas, temperature and the number of moles of the gas. These are related to 'R' - the Ideal Gas Law constant. T.
Charles' Law Problems (DOC 28 KB) Charles and Boyles' Law Problems Worksheet (DOC 26 KB) Gas Laws Pressure, Volume, Temperature Problems (DOC 24 KB) Air Bag Questions Warm Up (DOC 35 KB) Sketch the Relationships for an Ideal Gas Warm up (DOC 42 KB) Combine Gas Law Worksheet (DOC 24 KB) Density and Formula Mass Conversions of Ideal Gases (DOC ...
Solutions to the Ideal gas law practice worksheet: The ideal gas law states that PV=nRT, where P is the pressure of a gas, V is the volume of the gas, n is the number of moles of gas present, R is the ideal gas constant, and T is the temperature of the gas in Kelvins. Common mistakes: • Students express T in degrees celsius, rather than Kelvins.
Lesson Worksheet: The Ideal Gas Law. In this worksheet, we will practice using the ideal gas law, which relates the pressure, volume, quantity, and temperature of an ideal gas. What volume of oxygen at 423.0 K and a pressure of 127.4 kPa is produced by the decomposition of 129.7 g of B a O 2 to B a O and O 2?
Chapter 11 Ideal Gas Law Worksheet 2 - Density and Molar Mass via Ideal Gas Law 1. What is the density of carbon tetrachloride vapor at 714 torr and 125°C? 2. Find the molar mass of a gas that has a density of 1.18g/L at 25°C and 1 atm? 3. Exactly 250 mL of a gas at STP weighs 0.291g. The composition of the gas is as follows: C, 92.24%, H ...
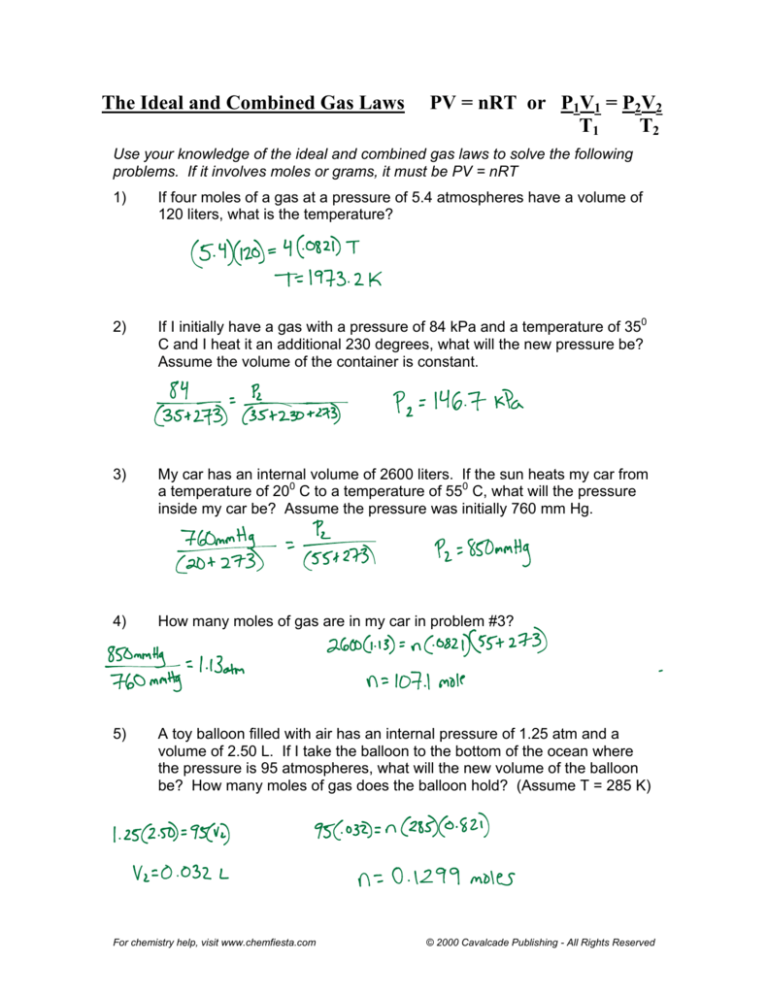
The Ideal and Combined Gas Laws PV = nRT or P 1V 1 = P 2V 2 T 1 T 2 Use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be PV = nRT 1) If four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature?
Charles' Law - relationship between volume and temperature; Avogadro's Law - relationship between moles and volume . A sample of hydrogen gas has a volume of 8.56 L at a temperature of 0 o C and a pressure of 1.5 atm. Calculate the moles of hydrogen present in the sample. 0.57 moles . How would this answer change if the gas had been helium?
Some of the worksheets displayed are Ideal gas law name chem work 14 4 Gas laws work Ideal gas law work pv nrt Ideal gas law practice work 2 Work 7 Ideal gas law practice work 9 23 combined gas law and ideal gas law wkst Mixed gas laws work. A sample of 4 25 moles of. R 008206 L atm mol K. The value of R varies with the units chosen.
View Ideal Gas Law Worksheet.docx from PHYSICS 1 at Pebble Hills High Schools. Name _ Period _ Ideal Gas Law Solve the following problems using the ideal gas law. 1. If I have 4 moles of a gas at a
Revised!CS7/15/13!!!!! ! !!!!!©LaBrake!&!Vanden!Bout!2013! Department of Chemistry University of Texas at Austin ! Gas$Laws$-$Supplemental$Worksheet$
































0 Response to "42 the ideal gas law worksheet"
Post a Comment