43 redox reactions worksheet with answers
Worksheet 5 balancing redox reactions in acid and basic solution balance each half reaction in basic solution. 2 nabr cl 2 2 nacl br 2 b. B balance the oxygen atoms with h 2 o. No no 3 6. C balance the hydrogen atoms with h d in a basic medium add one oh to each side for every h step 4. Redox Reactions Exercise With Solutions.
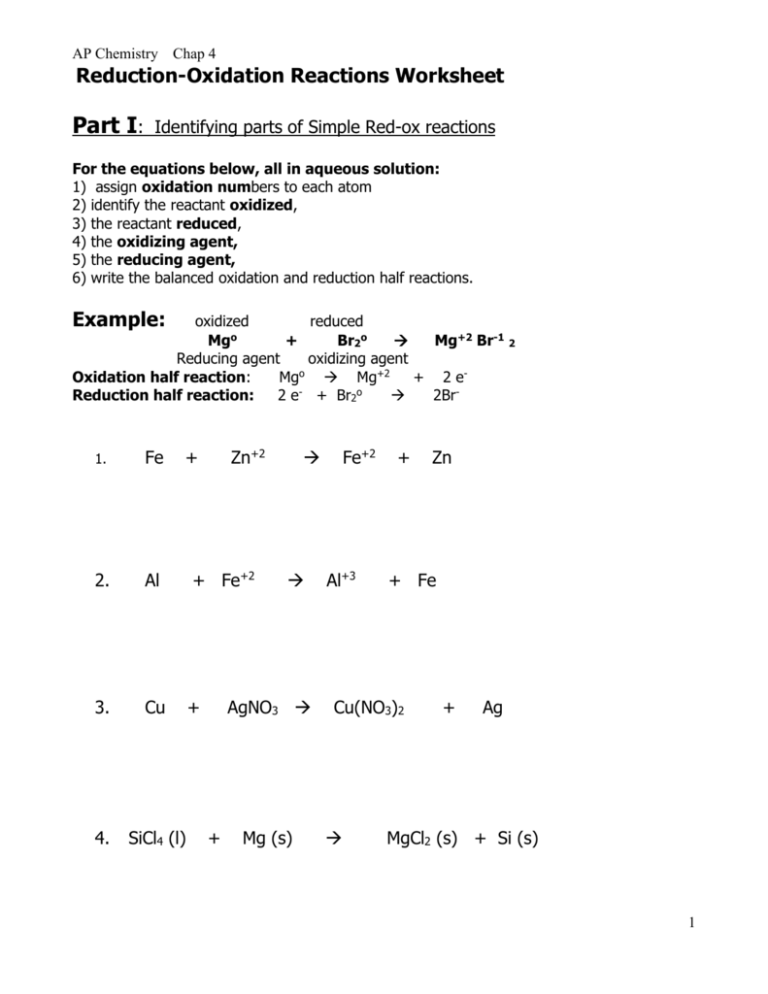
Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
24. In the reaction Mg+Cl2!MgCl2, the correct half-reaction for the oxidation that occurs is A. Mg+2e !Mg2+ B. Cl2 +2e !2Cl C. Mg !Mg2+ +2e D. Cl2!2Cl +2e 25. The reaction that takes place in a chemical cell is best classi ed as A. fusion B. redox C. transmutation D. cracking 26. Which equation represents the half-reaction that takes place at ...

Redox reactions worksheet with answers
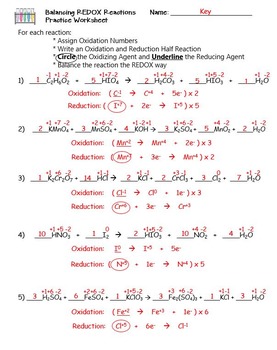
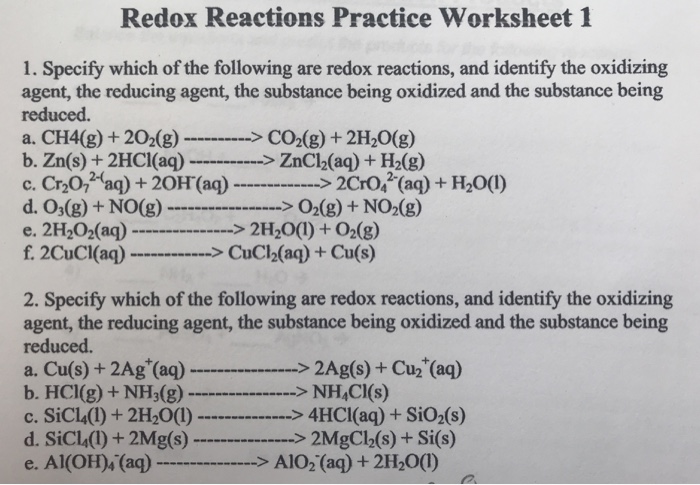
For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO. 3) 2KMnO4 + 5KNO2 + 3H2SO4 ( 2MnSO4 + 3H2O + 5KNO3 + K2SO4. ... Oxidation Reduction Worksheet Answers ...
Mno 2 mn 2o 3 balance each redox reaction in acid solution using the half reaction method. Redox reactions worksheet answers. A redox reaction always involves a. Oxidation is associated with electron loss helpful mnemonic. H 2o 2 cr 2o 7 2 o 2 cr 3 9. Determination of activity of some metals by reaction with hydrogen ion doc 28 kb redox ...
reaction and reduction half-reaction, identify the oxidizing and reducing agents and any spectator ions. Half - Reactions Homework ... Balance the following redox reactions by the half-reaction method, rewriting the balanced equations below the given unbalanced equation. Show your work below each reaction and
Redox reactions worksheet with answers.
Questions pertaining to redox reactions. 1 in combination with nonmetals o n. Cr 2o 7 2 cr3 5. No no 3 6. Balancing redox reactions worksheet 1. Clo3 cl æ cl2 clo2. 1 in all compounds 2. A change in phase. H 2o 2 cr 2o 7 2 o 2 cr 3 9. Choose a method and complete questions 12 13.
Some of the worksheets below are Redox Reactions Worksheets useful trick to help identify oxidation and reduction step by step guide to balance any Redox Equations explanation of Oxidation reduction oxidizing agent reducing agent and rules for assigning an oxidation number. Predicting redox reactions using the half reaction table 1. MnO4 - C2O4 2-.
Write balanced equations for the following redox reactions: a. 2 NaBr + Cl 2 2 NaCl + Br 2 b. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 in acidic solution c. 5 CO + I 2 O 5 5 CO 2 + I 2 in basic solution ; Write balanced equations for the following reactions: a. Cr(OH) 3 + Br 2 CrO 4 2-+ Br-in basic solution 10 OH-+ 2 Cr(OH) 3 + 3 Br 2 2 CrO 4 2-+ 8 H 2 O ...
! 207! Chapter12:!OxidationandReduction.!! Oxidation)reduction(redox)reactions. At!different!times,!oxidation!and!reduction!(redox)!havehaddifferent,but ...
The Redox Reaction Worksheet with Answers from A+Plate has been proven to work by a group of dietitians. It provides you with the answers that you are looking for. This helpful tool will help you determine whether or not a certain food or drink has or does not contain Redox reactions, which can be harmful for your body.
Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 K2CO3 K +1 C 4 O -2 c. NO3- N____+5_____ O____-2_____ HIO4 H +1 I +7 O -2 For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of ...
Recognizing redox reactions worksheet answers. 3mg n2 mg3n2 5. Balance each of the following half cell reactions. Suited for student in y10 and y11. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a. Redox reactions are a chemical reaction in which electrons are exchanged through oxidation and reduction.
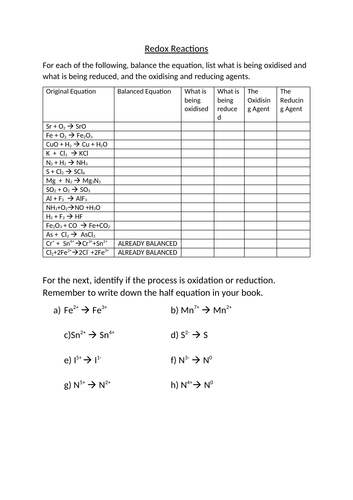
Balancing redox reactions in basic solution. If the redox reaction was carried out in basic solution (i.e. alkaline conditions), then we have to put in an extra step to balance the equation. The steps for balancing redox reactions in basic solution are: Identify the pair of elements undergoing oxidation and reduction by checking oxidation states
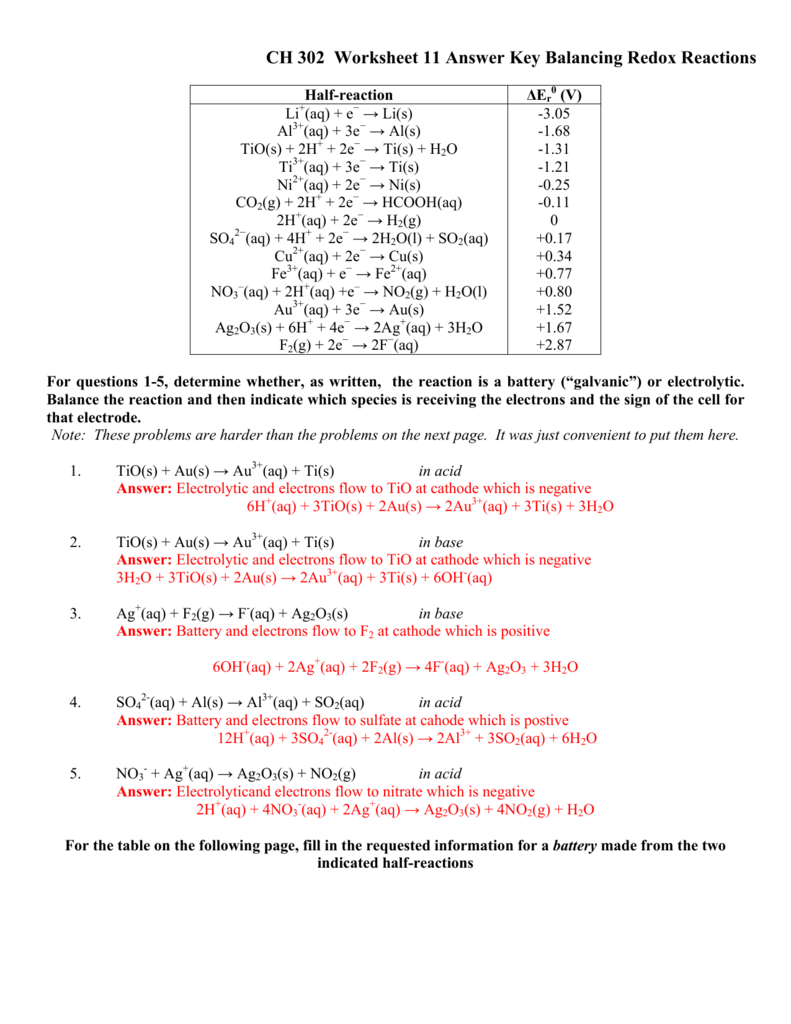
3. Balance the spontaneous redox reaction below. A spontaneous reaction is a reaction that occurs: 1) by a driving force that favors the product, 2) the free energy of the product is lower than the free energy of the reactant, and/or 3) occurs without any outside 'help' such as electrolysis. Identify the entities reduced and oxidized.
Worksheet # 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution. 4. Cr 2O 7 2 - → Cr3+ 5. NO → NO 3-6. SO 4 2- → SO 2 7. MnO 2 → Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. 8. H 2O 2 + Cr 2O 7 2- → O 2 + Cr 3+ 9. TeO 3 2-+ N 2O 4 → Te + NO 3-10 ...
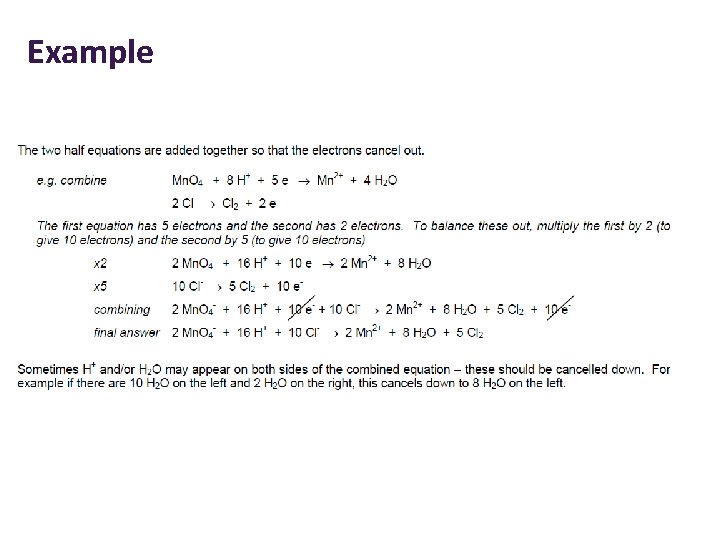
Balancing Redox Equations Method 2: Half-reaction method 1. Divide the skeleton reaction into two half-reactions, each of which contains the oxidized and reduced forms of one of the species 2. Balance the atoms and charges in each half-reaction - Atoms are balanced in order: atoms other than O and H, then O, then H
Chapter 20 Worksheet: Redox I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO 2. 2Li + S Li2S 3. 2Cs + Br2 2CsBr 4. 3Mg + N2 Mg3N2 5. 4Fe + 3O2 2Fe2O3 6. Cl2 + 2NaBr 2NaCl + Br2 7. Si + 2F2 SiF4 8. 2Ca + O2 2CaO 9.
WS # 4 Balancing Redox Reactions . Balance each of the following half-cell reactions. (In each case assume that the reaction takes place in an ACIDIC solution.) Also, state whether the reaction is oxidation or reduction. 1.
admin November 18, 2019. Some of the worksheets below are Redox Reactions Worksheets, useful trick to help identify oxidation and reduction, step by step guide to balance any Redox Equations, explanation of Oxidation, reduction, oxidizing agent, reducing agent and rules for assigning an oxidation number, …. Once you find your worksheet (s ...
Redox Reactions Answer Key Redox Reactions CHEM 10 Review Worksheet The questions on this worksheet are both Chem 10 and Chem 11 level questions. The reaction that takes place in a chemical cell is best classi ed as A. Redox Reactions Worksheet Pdf Printable worksheets are a valuable lecture room tool. Oxidizedreducing agent O0 to O2-.
Predicting redox reactions using the half reaction table 1. Redox reactions worksheet. Cr 2o 7 2 cr3 5. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. In the reaction 2k cl2 2kcl the species. In the reaction al0 cr3 al3 cr0 the reducing agent is a. Ws 4 balancing redox reactions. 3mg n2 mg3n2 5. 2cs br2 2csbr 4. 5 2 customer reviews.
Worksheet 1 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-d. NO 2-e. LiH f. Fe 3 O 4 Hint; Identify the species being oxidized and reduced in each of the following reactions: a. Cr + + Sn 4+ Cr 3+ + Sn 2+ b. 3 Hg 2+ + 2 Fe (s) 3 Hg 2 + 2 Fe 3+ c. 2 As ...
Balancing redox reactions worksheet 1 balance each redox reaction in. In each case assume that the reaction takes place in an acidic solution also state whether the reaction is oxidation or reduction. In which substance is the oxidation number of nitrogen zero. 2ca o2 2cao 9. There are many methods available for balancing redox reactions a ...
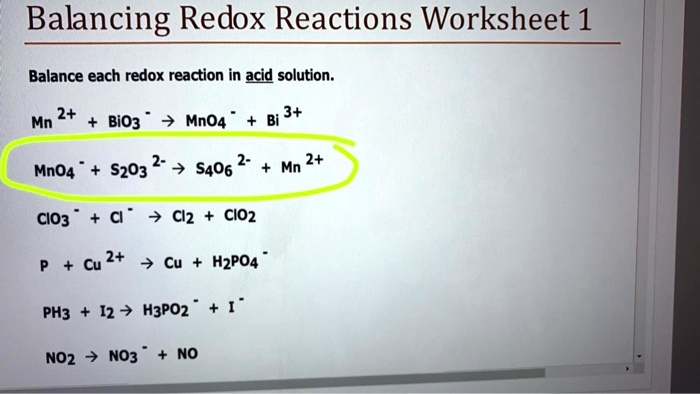
Balancing Redox Reactions Worksheet 1 Balance each redox reaction in . acid. solution. Mn 2+ + BiO3 -Æ MnO4 -+ Bi 3+ MnO4 -+ S2O3 2- Æ S4O6 2- + Mn 2+
Balancing redox reactions worksheet 1 answer key the following redox reaction takes place in acidic solution. You can in a half reaction but remember half reactions do not occur alone they occur in reduction oxidation pairs 2 here are the correct half reactions. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2 clo3 cl æ cl2 clo2.
Balancing redox reactions in basic solution. Answers to practice problems 1. Mg mg2 2e d. Balancing redox reactions worksheet 1 balance each redox reaction in. Balancing redox reactions chem 1a b steps for balancing redox reactions with the reaction method. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a.
Balancing REDOX Reactions: Learn and Practice Reduction-Oxidation reactions (or REDOX reactions) occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge. We can "see" these changes if we assign oxidation numbers to the reactants and products.




























0 Response to "43 redox reactions worksheet with answers"
Post a Comment