43 polar and nonpolar bonds and molecules worksheet answers
What is polar, nonpolar bonds and dipole moment? | Forum A polar molecule is a molecule where one end has a positive electrical charge and the other end has a negative charge due otherwise, the molecule is considered nonpolar; Molecule which has no separation of charge, so no positive or... Chapter 12 Review 1: Covalent Bonds and Molecular Structure 2) How are nonpolar covalent bonds different from covalent bonds, and what types of elements combine to form each? polar – electrons are share unequally ...5 pages
What is polar/nonpolar? The difference between polar and nonpolar bonds as follows. Polar bonds. - Ionic bond formation takes place between atoms of strongly electropositive and strongly electronegative A molecule in which there is a little division of charge in the chemical bonds, so that one part of the molecule has a...

Polar and nonpolar bonds and molecules worksheet answers
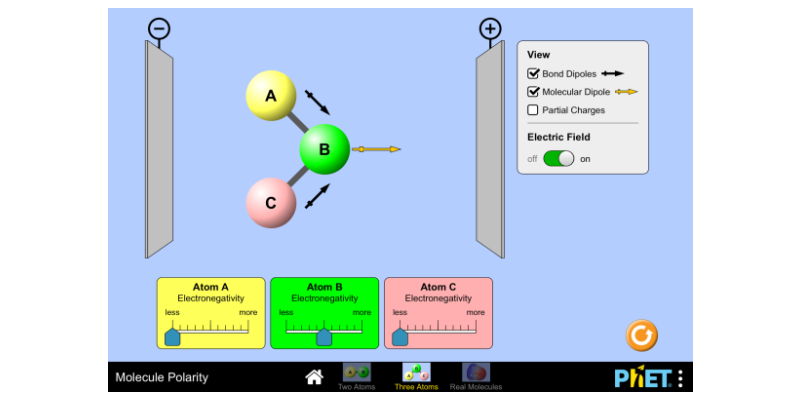
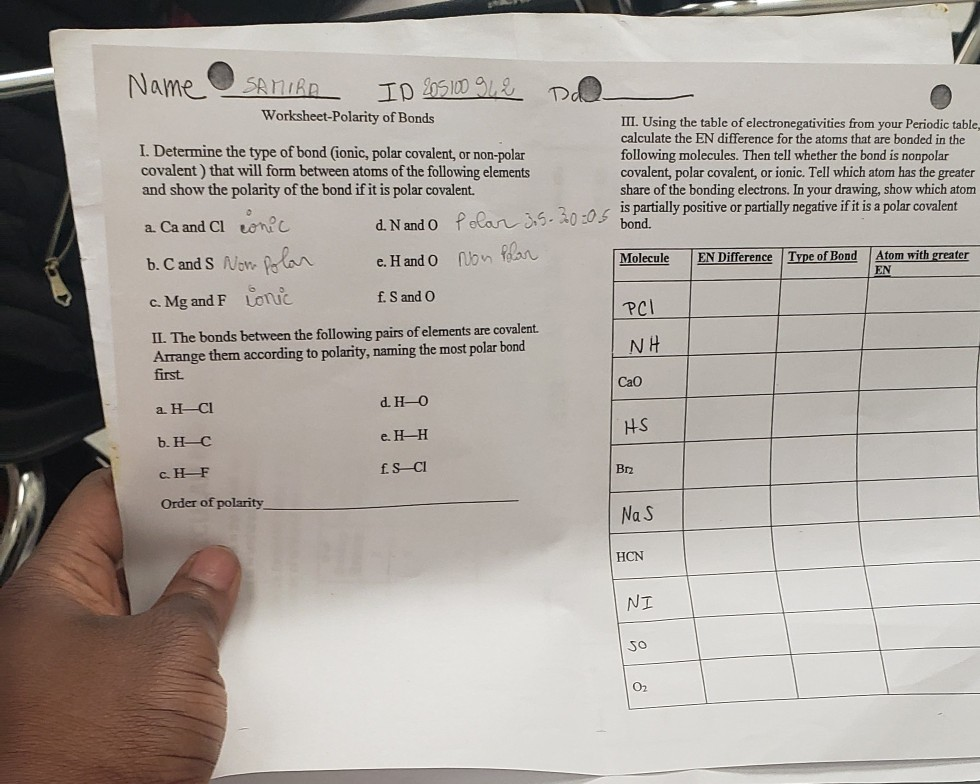
Information on molecular polarity for An Introduction to Chemistry by... Molecular Polarity. When there are no polar bonds in a molecule, there is no permanent charge difference between A molecule can possess polar bonds and still be nonpolar. If the polar bonds are evenly (or symmetrically) distributed, the bond dipoles cancel and do not create a molecular dipole. Polar vs. Nonpolar Polar and nonpolar compounds. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density Nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. chan(sillt Narot f. S ClbO5 chan(sillt. Narot. Worksheet-Polarity of Bonds. I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form.2 pages
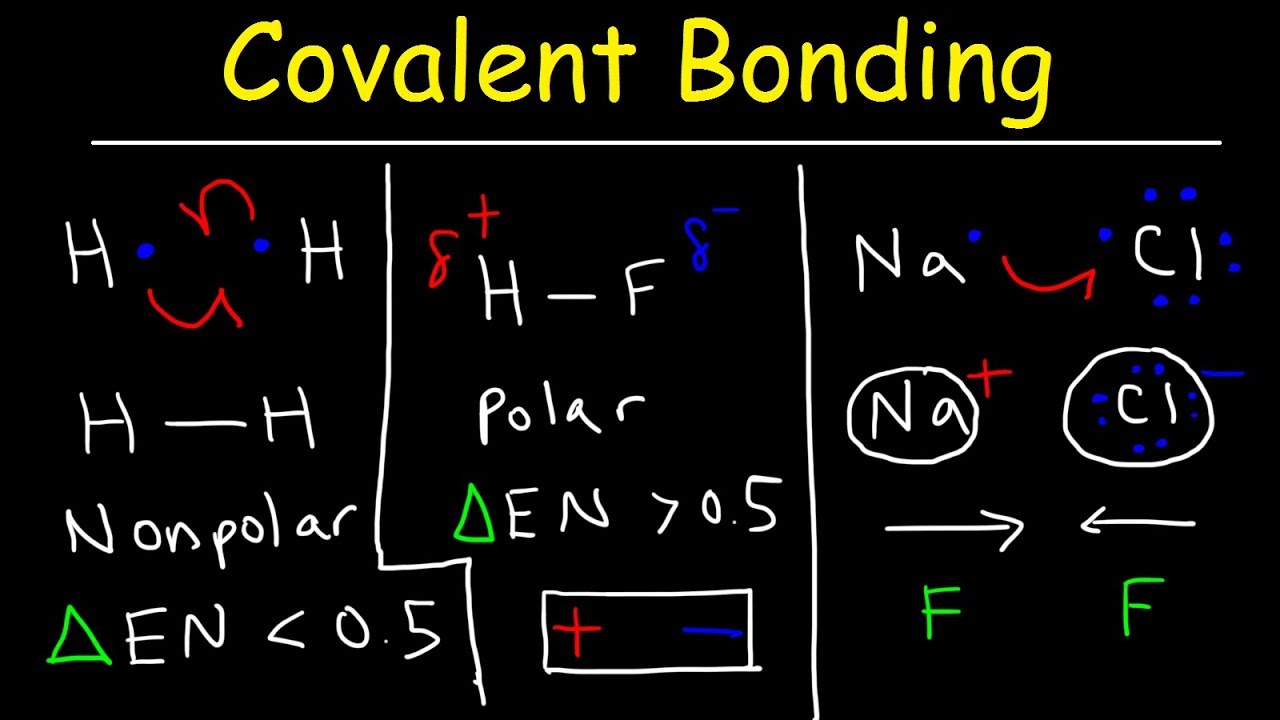
Polar and nonpolar bonds and molecules worksheet answers. Shapes of Molecules | Polar and Nonpolar Covalent Bonds A molecule must contain polar bonds in order for the molecule to be polar, but if the polar bonds are aligned exactly opposite to each other, or if they The polarity of a molecule has a strong effect on its physical properties. Molecules which are more polar have stronger intermolecular forces between... 4.4: Polar and Non-polar Covalent Bonds - Chemistry LibreTexts Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between When a molecule's bonds are polar, the molecule as a whole can display an uneven distribution of nonpolar; polar; ionic. Key Takeaways. Covalent bonds between different atoms have different... Questions on polar and nonpolar molecules | Physics Forums 1. Can a molecule have only nonpolar bonds and have a dipole? My first thought was no, that only molecules made of the same element have nonpolar bonds. Answers and Replies. Nov 23, 2009. Related Threads on Questions on polar and nonpolar molecules. How to Determine if a Molecule is Polar or Non-Polar: Check Now Examples of polar and nonpolar molecules. Polar molecules: Water, alcohol, Sulphur dioxide, ammonia, ethanol, hydrogen sulfide, bent molecules (those with a significant bond angle) in general. Non-polar molecules: Hydrocarbons (gasoline, toluene), homo-nuclear diatomic molecules (O2, N2...
Polar vs. Nonpolar Bonds — Overview & Examples - Expii In a polar molecule, the more electronegative atom will have a higher density of electrons near it. Since electrons carry a negative charge, this atom will also have a partial Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. Polar vs Nonpolar Molecules- Definition, 7 Key Differences, Examples Polar molecules usually have a higher boiling and melting point as well as a high surface tension as polar linkages are considerably stronger Nonpolar molecules do not show any orientation in an electric field as no electrostatic interactions occur between nonpolar molecules and the electric field. Overview of Biomolecules Book - Charles E. Schmidt College ... typical organic molecules. They are the same types of molecules, react in the same ways, and obey the same physical laws. B. Composition and Structure Biomolecules contain mainly carbon, which behaves as it always does in organic compounds, forming 4 bonds, usually with a tetrahedral arrangement. (PP 2) The Polar and Nonpolar Molecules | Polar and Nonpolar Chemical Bonds Polar and nonpolar molecules are the two broad classes of molecules. Polarity describes the distribution of electrical charge around a molecule. Understanding and identifying polar and nonpolar chemical bonds makes it easier to understand polar molecules.
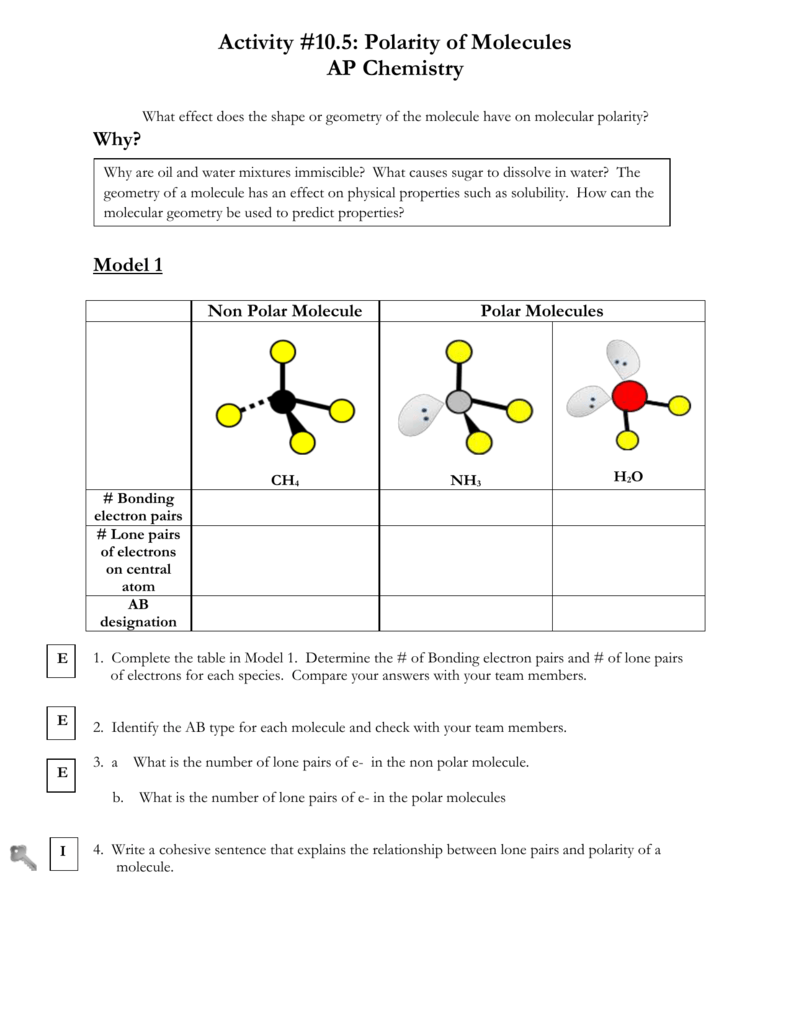
Structure of Polar and Nonpolar Molecules.pdf - Crestwood ... Structure of Polar and Nonpolar Molecules ... Which molecules in this illustration have polar covalent bonds? ... Transparency Worksheet 26 ...2 pages Distinguish between polar and nonpolar bonds. - Brainly.ph Give 3 examples of molecules that are polar and nonpolar. krystelrosee krystelrosee. Answer: •Water is polar. •Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Proteins, Carbohydrates, and Lipids covalent bonds Oligosaccharides: three to 20 monosaccharides Polysaccharides: ... Lipids are nonpolar hydrocarbons. When sufficiently close together, weak but ... Non polar and amphiphatic molecules . 3 Nucleic Acids and the Origin of Life . Difference Between Polar and Nonpolar Bonds Main Difference - Polar vs Nonpolar Bonds. Both polar bonds and non-polar bonds are two types of covalent bonding between atoms. In covalent bonding, the electrons are shared between the two atomic species involved, instead of a complete giveaway or acceptance of electrons.
Polar And Nonpolar Molecules Worksheet , Jobs EcityWorks polar-and-nonpolar-molecules-worksheet 2/6 Downloaded from test.classygroundcovers.com on May Worksheet Answers Electronegativity Worksheet Answers Quiz & Worksheet - Nonpolar Download File PDF Polar And Nonpolar Molecules Worksheet Polar bonds supplemental work...
Polar vs. Non-Polar Bonds & Molecules | ChemTalk Everything you need to know about polar bonds, non-polar bonds, polar molecules, and non-polar molecules with helpful examples & diagrams. To understand the difference between polar and non-polar bonds, it is essential to comprehend electronegativity.
Worksheet - Polar and Nonpolar Molecules Jones 2014 | PDF Worksheet on Polar and Non Polar Molecules. Copyright: © All Rights Reserved. Flag for inappropriate content. Determine if the molecules listed below are polar or nonpolar. To be polar, a molecule must: have polar-covalent bonds AND not be symmetrical.
Differences Between Polar & Nonpolar in Chemistry | Sciencing The difference between polar and nonpolar bonds stems from the difference in electronegativity of the atoms Nonpolar compounds either have no polar bonds or contain symmetrical polar bonds. Why Does Polarity Matter? Chemical polarity plays a huge role in how different molecules interact.
Which molecule contains both polar and nonpolar covalent bond? Polarity is resulting from unequal sharing of electrons. There are three sigma bonds present in hydrogen peroxide. Two of them are between hydrogen The other one is between two oxygen atoms where there is no unequal sharing of electrons as they have same dipole moment. So it is non polar.
Jamila-Polar and Nonpolar Molecules Worksheet.pdf - Jamila... Jamila Reid April 28, 2021 Polar and Nonpolar Molecules Worksheet Instructions: Determine if the molecules listed below are polar or nonpolar. To be polar, a molecule must have polar-covalent bonds AND not be symmetrical. Oxygen gas, O 2 Electronegativities...
Which molecule has polar bonding and is non-polar? - Quora Polar bond but non polar molecules: When there are no polar bonds in a molecule, there is no permanent charge difference between one part of the molecule and another, and the molecule is nonpolar.
Polar Covalent Molecules Circle the pairs of atoms below that would form a nonpolar covalent bond? C&H - (N&c ) H&O. P&H. F&S. AENTO TEN-O DENTO A ENZO A.5 pages
Classifying Chemical Bonds - SAS Diatomic molecules have nonpolar covalent bonds. There are seven diatomic molecules: H2, N2 Collect and check the Types of Bonds worksheet and Comparing Ionic, Polar Covalent, and Nonpolar Covalent Bonding Go over the answers with the class, and provide further instruction as necessary.
Difference Between Polar and Nonpolar Generally, polar molecules and polar solvents possess large dipole moment values. Nonpolar molecules are formed when electrons are shared equally between atoms of a molecule. The article discusses the difference between polar and nonpolar bonding, compounds and solvents in detail.
Polar and Nonpolar bonding note Polar and Non-Polar Covalent Bonds If an electron pair is shared equally between two bonding atoms, the bond is called nonpolar covalent. Examples: diatomic elements such as H2, N2, Cl2 where the nuclei attract electrons with equal force. When the sharing of bonding valence electrons is not...
Examples of Polar and Nonpolar Molecules Key Takeaways: Polar and Nonpolar. In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons...
Polar and nonpolar molecules worksheet answer key - Junior ... Covalent titles have some characteristics that depend on the identity of the atoms participating in the bond. Two features are length of bond and polarity ...2 pages
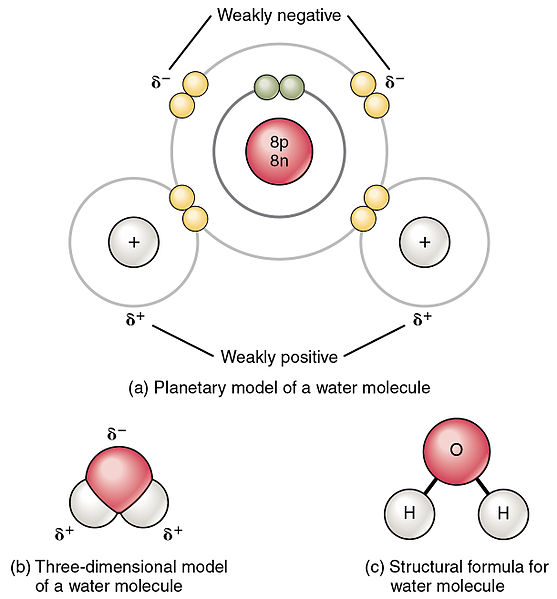
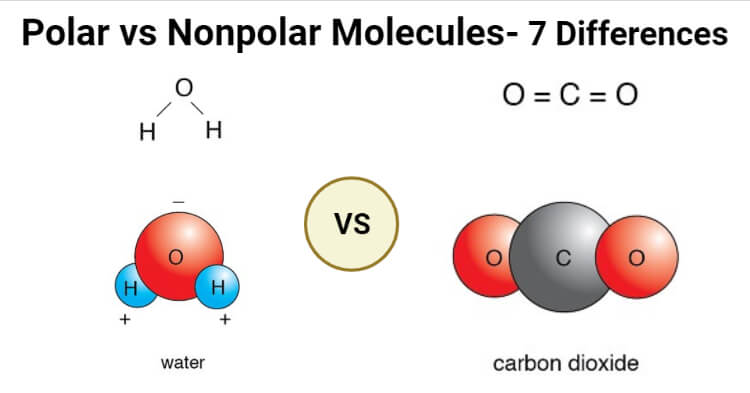
Which of these molecules contain polar bonds, and... - Brainly.com Carbon(IV) oxide is nonpolar because CO₂ is linear molecule and the oxygen atoms are symmetrical (bond angles 180°). Water is polar because of the bent shape of the molecule. Use your points to get answers right away. You set the number of points you think your question is worth.
Which of these molecules contain polar bonds, and which contain... water and CO2 contain polar bonds. *keep in mind that this does not indicate whether or not the molecule is polar or not since that has to do with the molecule's VESPER shape. ie. Get a free answer to a quick problem. Most questions answered within 4 hours.
Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or... This video provides a fast way for you to determine if a molecule is polar or nonpolar. It provides examples so you can quickly distinguish nonpolar...
Polar vs Nonpolar - It's all about sharing, on an atomic level Polar bonds (with polarity) are much weaker, and they require less energy to break their bonds. This means that polar molecules can more easily Both polar and nonpolar are a type of covalent bond which is where atoms bond by sharing electrons. Covalent bonding typically occurs with nonmetallic...
Polar And Nonpolar Molecules Worksheet Answers janneya - Coub Polar And Nonpolar Molecules Worksheet Answers janneya. molecules worksheet answers. polar and nonpolar molecules crash course worksheet answers. structure of polar and nonpolar molecules worksheet answers. polar nonpolar and ionic bonds worksheet answers 28d79c4b43.
What is a Diatomic Element?| Diatomic Elements List ... Dec 13, 2021 · When water is a liquid, the hydrogen bonds between two molecules of water are fluid. When water solidifies into ice, so do the hydrogen bonds. As they become more rigid, more space is trapped ...
Ionic and Covalent Bonds - Chemistry LibreTexts Sep 13, 2020 · This creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the middle. Both of these bonds are important in organic chemistry. Ionic bonds are important because they allow the synthesis of …
chan(sillt Narot f. S ClbO5 chan(sillt. Narot. Worksheet-Polarity of Bonds. I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form.2 pages
Polar vs. Nonpolar Polar and nonpolar compounds. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density Nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal.
Information on molecular polarity for An Introduction to Chemistry by... Molecular Polarity. When there are no polar bonds in a molecule, there is no permanent charge difference between A molecule can possess polar bonds and still be nonpolar. If the polar bonds are evenly (or symmetrically) distributed, the bond dipoles cancel and do not create a molecular dipole.






























0 Response to "43 polar and nonpolar bonds and molecules worksheet answers"
Post a Comment