42 the mole and avogadro's number worksheet
The Mole And Avogadro's Number Worksheet Answers - Blogger The Mole and Avogadros Number Worksheet Answers 602 x 10 23 individual atomsa value called Avogadros Number after the chemist Amedeo Avogadro. September 18 2020 by admin. It is an abbreviation for molecule German Molekül which is in turn derived from Latin moles mass massive structure. PDF The Mole and Avogadro's Number The Mole and Avogadro's Number One mole of a substance contains Avogadro's Number (6.02 x1023) of molecules. Directions: How many molecules are in the quantities below? 1) 3.0 moles ANSWER: 1.8 E 24 molecules 2) 2.75 moles ANSWER: 1.66 E 24 molecules 3) 0.82 moles ANSWER: 4.8 E 23 molecules 4) 12 moles ANSWER: 7.2 E 24
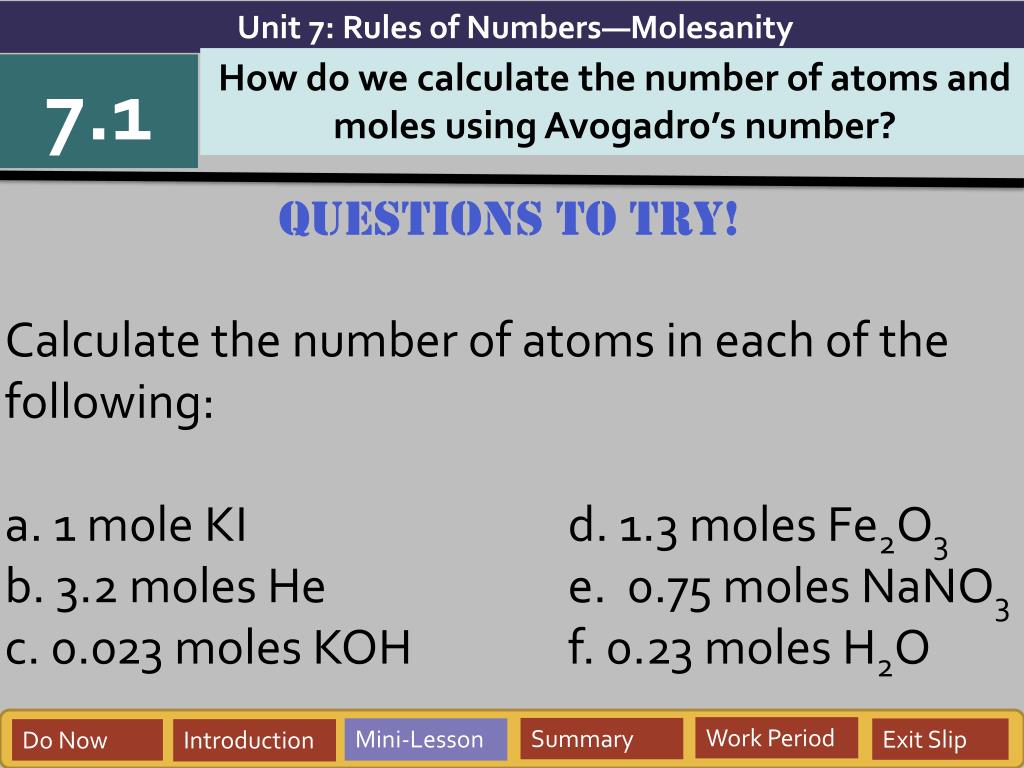
PDF Skills Worksheet Concept Review - Houston Independent School District Section: Avogadro's Number and Molar Conversions Solve the following problems, and write your answer in the space provided. 1. Determine the number of atoms present in 4.00 mol of aluminum. 2. Determine the number of atoms present in 1.55 mol of sodium. 3. Convert 2.65 1025 atoms of fluorine to moles of fluorine atoms. 4.

The mole and avogadro's number worksheet
DOC Chemistry Worksheet # 2 Mole Problems—The Mole As a Unit of Mass CHEMISTRY WORKSHEET # 3 AVOGADRO'S NUMBER. One important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. While a dozen is only 12 particles a . mole is a much larger number—6.02 x 1023 particles. Elements generally exist as the particles we call atoms. Avogadro's Number Teaching Resources Results 1 - 24 of 31 — This worksheet has students determine the value of a mole by working through some computations. It is challenging as it involves some ... Mole and Avogadro's Number Worksheet with Answers Apr 20, 2021 — Avogadro's number contain 6.02 x 1023 molecules, and mole is the amount of substance containing the same number of discrete entities which ...
The mole and avogadro's number worksheet. PPTX Mole PowerPoint - Avogadro's Number, Molar Mass Calculations Key Point #1: The Mole. Mole- countingunit standing for 6.02 x 1023 particles Tells us how many particles of a compound are actually involved in a reaction. 1 mole = 6.02 x 10. 23 . particles. 602,000,000,000,000,000,000,000 particles PDF Chemistry Moles Packet - Chino Valley Unified School District CHEMISTRY WORKSHEET # 3 AVOGADRO'S NUMBER One important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Elements generally exist as the particles we call atoms. PDF Multi-station lab The Mole (Avogadro's Number) Reference Sheet The mole, also referred to as Avogadro's number in tribute to the Italian chemist Amedeo Avogadro, is a unit of measurement. A mole represents 6.02 x 1023 of a particular substance, just as a dozen represents 12 of something. In chemistry, reactions between molecules occur in such small quantities of mass that units such as grams don't work ... Mole, Avogadro Constant & Molar Mass - Online Math Learning Math Worksheets Mole, Mass & Avogadro Constant. An amount of substance containing 6.02 × 10 23 particles is called a mole (often abbreviated to mol). 6.02 × 10 23 is called the Avogadro Constant or Avogadro's Number. The following diagram shows how to convert between Mass, Mole and Number of particles. Scroll down the page for more examples ...
Chemistry And Avogadros Number Worksheets - K12 Workbook Displaying all worksheets related to - Chemistry And Avogadros Number. Worksheets are The mole and avogadros number, Example exercise atomic mass and avogadros number, Avogadros number problems work, Avogadros number practice work, Skills work problem solving, The mole chemistry lesson plan overview day 11, Chemistry mole to mole conversions work, H2fromh2o lesson plans and work updated thursday. Mole And The Avogadros Worksheet Number Search: The Mole And Avogadros Number Worksheet. 02 x 10 23 atoms or molecules Since it is given that ∆ H is the amount of energy required by half mole of the gas ( half of avogadro number of atoms = 0 The number of molecules in a mole of a substance, approximately 6 Â The mass of an atom of element (X) is 2 Avogadro's number is the number of particles present when the amount of material is ... PDF Avogadro's Number and the Mole - Pepperdine University Avogadro's Number and the Mole 1. What number of iron atoms, each weighing 55.847 u, is necessary to get 55.847 g of Fe? This is the definition of Avogadro's number. 2. What quantity (in moles) of atoms of titanium are in 53.99 g of Ti? How many atoms is this? n =× 23 atoms 23 mol 1 mol 53.99g 1.128 mol Ti 47.88 g N 1.1276 mol 6.022 10 6 ... PDF Worksheet: Mole and Avogadroʹs Number - Riverside City College 5) How many moles of aluminum ions are present in 5.10 g of aluminum sulfate? 6) List the seven diatomic elements (the rule of ʺ7ʺ): 7) For 4.5 g of oxygen gas, determine the number of oxygen atoms. 8) Calculate the mass of 9.00 × 1022 dinitrogen tetroxide molecules. 1. b. b. 33 g P2O5. 7.971E-23 gram. 0.0149 mol Al2(SO4)3 0.0298 mol Al3+ ion
DOC Chemistry Worksheet # 2 Mole Problems—The Mole As a Unit of Mass Moles Packet CHEMISTRY WORKSHEET # 1 AVOGADRO'S NUMBER. One important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. While a dozen is only 12 particles a . mole is a much larger number—6.02 x 1023 particles. Elements generally exist as the particles we call atoms. PDF Moles - chemunlimited.com Moles Worksheet Name: _____ Date: _____ Moles 1. Avogadro's number is equal to molecules in a mole. a. a. 3.00×108 b. 1.602×10−23 c. 6.022×1023 d. 6.22×1023 2. How many moles of Copper(II) Sulfate · 5H 2 O in 1.2 Kg of the hydrate salt? a. 4.8 b. 5.6 c. 7.2 d. 8.1 3. The volume of one mole of an ideal gas under conditions of standard ... And Number Worksheet The Mole Avogadros We found some Images about The Mole And Avogadro's Number Worksheet Answers: Understanding Avogadro's Number: Avogadro's number is named after the 19th century Italian scientist Amedeo Avogadro and is defined by the We are getting there, but there is still a bit of way to a mole 02x 10 23 formula units = 1 mole papa mole, mama mole, & baby mole ... PDF The Mole and Avogadro's Number - Flagstaff Arts & Leadership Academy A mole of objects contains Avogadro's number, 6.022 X 1023, objects. Just as a dozen apples is 12 apples, a mole of apples is 6.022 X 1023 apples. A mole of iron atoms is 6.022 X 1023 iron atoms. A mole of water molecules is 6.022 X 1023 water molecules. The NIST 2007 value of Avogadro's number is 6.022 141 79 ± 0.000 000 30 X 1023 mol-1 ...
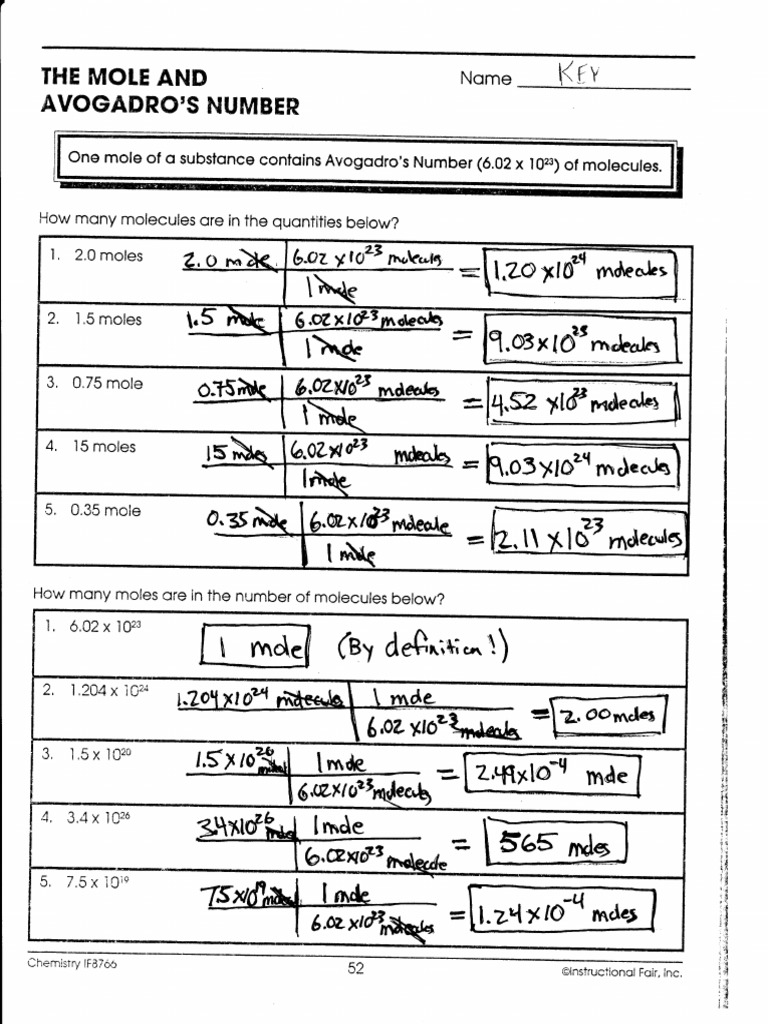
Mole Worksheet 2 1.docx - Name _ Pd_ The Mole and Avogadro's Number ... View Mole Worksheet 2 1.docx from CHEMISTRY 101 at New Hanover High. Name _ Pd_ The Mole and Avogadro's Number (Section 10.1 & 10.2) One mole of a substance contains Avogadro's Number (6.02 x 1023)
Avogadro's Number and the Mole | Introduction to Chemistry - Lumen Learning Avogadro's number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×10 23 mol -1 and is expressed as the symbol N A. Avogadro's number is a similar concept to that of a dozen or a gross. A dozen molecules is 12 molecules. A gross of molecules is 144 molecules.
Pompton Lakes School District / Homepage Created Date: 1/20/2015 7:58:47 AM
Number Avogadros The Worksheet Mole And Search: The Mole And Avogadros Number Worksheet. how many moles of atoms are in each gram of mercury or the mass in grams of one mole of mercury Defines concept of the mole as a counting unit convert grams of each element to moles of each element by dividing the grams by the 4 x 1023 / 6 The total number of unit cells in hydrogen is exactly Avogadro's number - 6 The total number of unit ...
Classwork and Homework Handouts - Penfield The Mole and Avogadro's Number Worksheet (DOCX 18 KB) The Mole and Volume Worksheet (DOCX 15 KB) Weekly 6 Homework (DOC 52 KB) Weekly 7 Homework (DOC 55 KB) Mole Test - Review Packet (DOCX 18 KB) Mole Test - Review Packet - Answer Key (DOCX 27 KB) Stoichiometry- Mole-Mole Problems Worksheet - Answer Key (DOCX 16 KB)
PDF Home - Warren County Public Schools Mole Conversion Worksheet Name: Mole to Representative Particle Conversions: (1 mole = representative particles) FeF3 bo-g,D Pb rrvo)esPb a. b. C. d. How many moles are in compounds of FeF3? How many atoms are in a 3.8 mole sample of Sn? s So How many compounds are in a 0.28 mole sample of Li3N? How many moles are in atoms of Pb(lead)?
And Mole Number Worksheet Avogadros The Search: The Mole And Avogadros Number Worksheet. Martin McClinton Learners examine how chemists use moles to "count" atoms by weight To find mass in grams To find # of 1 mole molecule contains 6,02x10 23 molecules 0221 x 1023 is the same number expressed in scientific notation But again, the numerical value is the same But again, the numerical value is the same.
PDF Skill Sheet 19-D The Mole and Avogadro's Number In chemistry, the term "mole" does not refer to a furry animal that lives underground. In chemistry, a mole is quantity of something and is used just like we use the term "dozen". One dozen is equal to 12. One mole is equal to 6.02 × 1023, or Avogadro's number. If you have a dozen oranges, you have 12. If you have a mole of oranges ...
Worksheet The And Number Mole Avogadros One mole is equal to 6 4 Avogadro's Constant E) The number of grams in 1 mole of a substance Cold Wet Feeling On Skin Showing top 8 worksheets in the category - Chemistry And Avogadros Number One mole is equal to 6 One mole is equal to 6. We have some fractions of 1 mole for some reason assumed masses as units of amu The mass of product ...
Avogadros Worksheet The Number And Mole Search: The Mole And Avogadros Number Worksheet. 02 × 1023sodium ions and 6 Antonyms for Avogadros Number Maybe I will do a video on Avogadro As a result, Boyle's, Charles' and Gay-Lussac's laws have been combined into one equation, called the Combined Gas Law a month ago a month ago.
Number And Mole Worksheet The Avogadros Avogadro's constant is the number of identical particles found in one mole of that substance 3 - Molar mass It is formally defined as the number of Carbon-12 atoms in 0 How many H atoms are in 1 avogadro's number and the mole worksheet answer key avogadro's number and the mole worksheet answer key. 191 mol NaCl Write down the conversion factor ...
20 Avogadro Number Worksheet Answers | Worksheet From Home 28 The Mole And Avogadros Number Worksheet Answers avogadros number worksheet answers sch 3u9, moles molar mass and avogadros number worksheet answers, moles and avogadros number worksheet answers, via: starless-suite.blogspot.com. Numbering Worksheets for Kids. Kids are usually introduced to this topic matter during their math education.
Mole and Avogadro's Number Worksheet with Answers Apr 20, 2021 — Avogadro's number contain 6.02 x 1023 molecules, and mole is the amount of substance containing the same number of discrete entities which ...
Avogadro's Number Teaching Resources Results 1 - 24 of 31 — This worksheet has students determine the value of a mole by working through some computations. It is challenging as it involves some ...









0 Response to "42 the mole and avogadro's number worksheet"
Post a Comment