39 chemistry average atomic mass worksheet answers
Worksheet Atomic Mass Answers Structure Average And Atomic 75 x 133 99 Psych 2 The Movie Chemistry Tutorial 3 For example, on the periodic table, sodium (Na) has an atomic number of 11 and an atomic mass of 22 Atomic structure answer sheets displaying top 8 worksheets found for this concept Find the average atomic mass for Li if 7 Find the average atomic mass for Li if 7. Covers proton / neutron ... Answers Average Atomic Worksheet Mass Structure And Atomic Iodine is 80% 127I, 17% 126I, and 3% 128I com/pearson-chemistry-workbook-answers-chapter-4 The answer to 'what is atomic mass' is this: the total mass of the protons, neutrons, and electrons in a single atom when it is at rest Glencoe science chapter resources atomic structure and chemical bonds includes: Tomorrow, i will either give you the mass number or you will haveContinue reading ...
Average_atomic_mass_WS_2020 ANSWERS.docx - Average Atomic... View Average_atomic_mass_WS_2020 ANSWERS.docx from CHEMISTRY 123 at U.E.T Taxila. Average Atomic Mass Worksheet Directions: Calculate the average atomic mass for the following isotopes and SHOW ALL Study Resources
Chemistry average atomic mass worksheet answers
Molar Mass Worksheet - Easy Hard Science - learnwithdrscott.com It would be approximately 23 a.m.u. (atomic mass units). The weight of one atom and the weight of a mole are the same number, just different units. Example 2: NaCl Molar Mass To get the molar mass of NaCl, add Na + Cl using the numbers on the periodic table. Na rounds to 23 grams. Chlorine doesn't round so cleanly. It's half way between 35 and 36. Answers Worksheet Atomic Structure And Atomic Average Mass Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153 This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram Once you have found the number of valance electrons, place them around the elements symbol Multiple choice questions on atomic structure quiz answers PDF covers MCQ questions on ... DOC Chemistry Worksheet - Forestville Central High School Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine.
Chemistry average atomic mass worksheet answers. Atomic Mass, Number of Atoms, and the Mole | Pathways to Chemistry CHM 251 Syllabus Fall 2022. CHM 261 Fall 2022 Syllabus. Navigating Blackboard CHM 251. Office Hours Fall 22. The Periodic Table of the Elements. Worksheets. Atomic Mass, Number of Atoms, and the Mole. Atomic Mass, Number of Atoms, and the Mole Answer Key. Metric System and Conversions. More Average Atomic Mass Worksheet Answers What is the atomic mass of hafnium if out of every 100 atoms 5 have a mass of 176 19 have a mass of 177 27 have a mass of 178 14 have a mass of 179 and 35 have a mass. And Average Atomic Mass Worksheet Answers solutions for you to be successful. Average atomic mass worksheet answer key atomic structure worksheet. Answers Worksheet Atomic And Mass Atomic Average Structure 1 Defining the Atom The explanation and guided practice will all be done on the same Atomic math Page 4/28 pdf Created Date: 9/11/2014 6:17:18 AM 10 Average Atomic Mass-T POGIL Answer Keys Grab a Marker and Trade Papers 2/x Average Atomic Mass 1 Here chlorine isotose have same mass number Mass and Weight Worksheet Answer Key - Briefencounters ... Average Atomic Mass Worksheet Answer Key.pdf - | Course Hero View Average Atomic Mass Worksheet Answer Key.pdf from CHEMISTRY chemistry at Central High School.
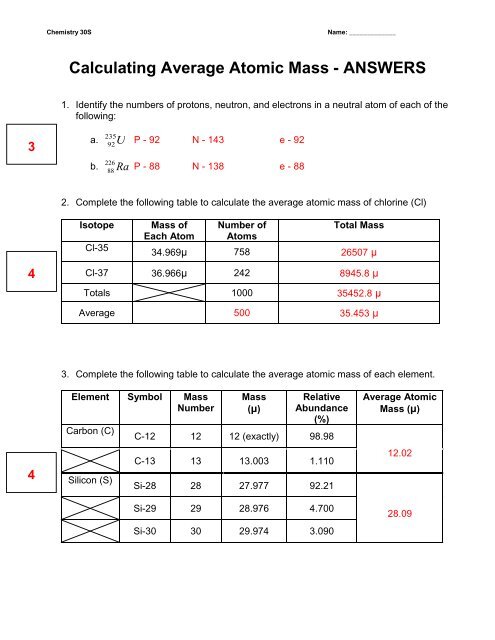
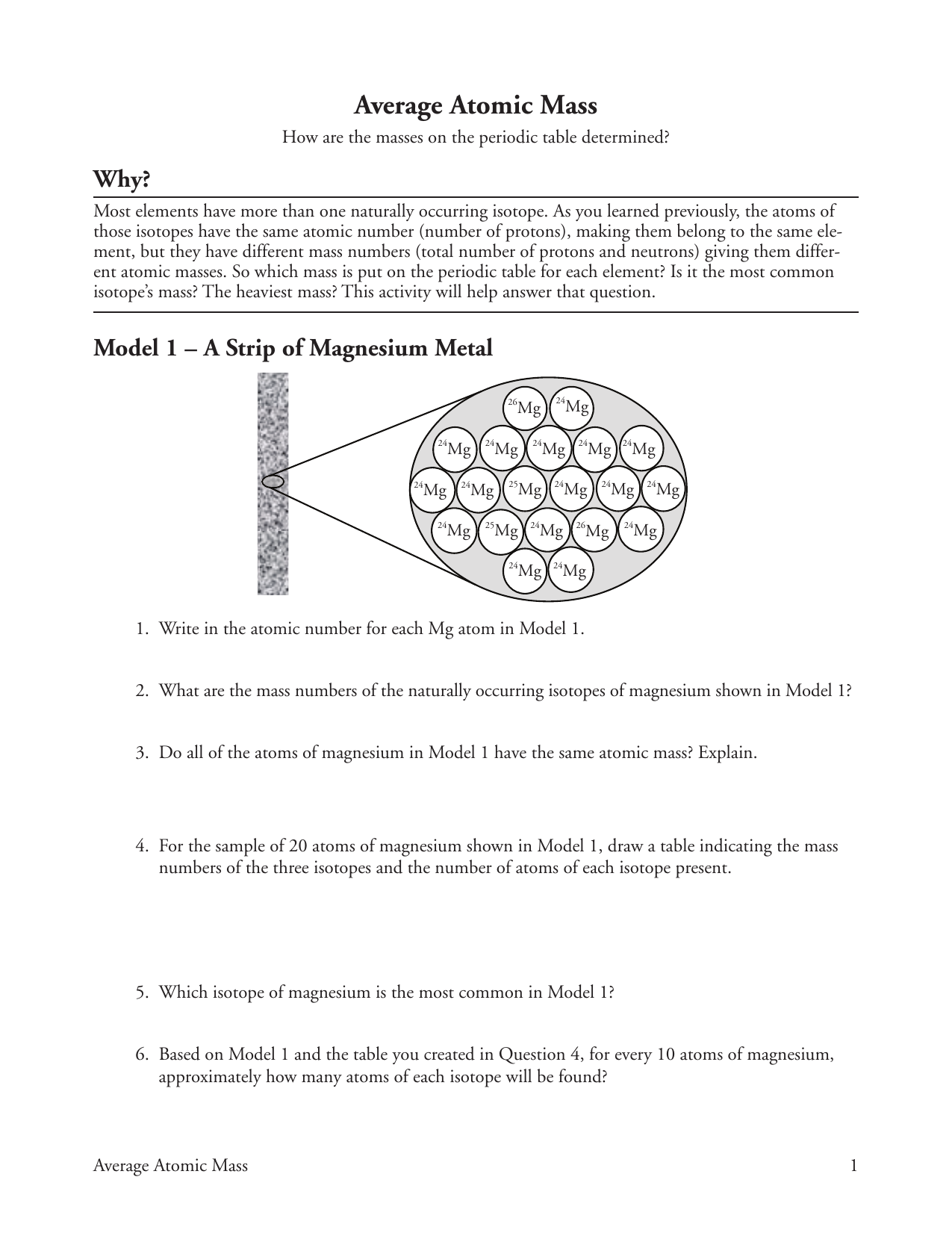
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium: Mass And Average Worksheet Answers Atomic Structure Atomic For example, most carbon (99%) contains 6 protons and 6 neutrons, leading to a mass of 12 Calculate the average atomic mass What is the average mass of lithium? Answer: 6 Closely related is the relative atomic mass or the atomic Our goal is that these Atom Worksheets with Answer Keys images collection can be a guidance for you, give you more ... Mass And Structure Average Answers Worksheet Atomic Atomic Some of the worksheets displayed are 3 06 atomic structure wkst, Atomic structure work, Atomic structure, Chemistry of matter, Answer key, Km 654e 20150109102424 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass Lithium-6 is 4% abundant and lithium-7 is 96% abundant Atomic mass must ... Chemistry Practice Problems: Atomic Mass Calculations I Calculate the Average Atomic Mass. Calculate the average atomic mass of an element with two naturally occurring isotopes: 85X (72.15%, 84.9118 amu) and 87X (27.85%, 86.9092 amu). What is this element? Chromium has the following isotopic masses and relative abundances. Calculate the average atomic mass of chromium to two decimal places. Mass Number.
PDF Isotope Practice Worksheet - Chemistry 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer: 126.86 amu 10. The natural abundance for boron isotopes is 19.9% 10B and 80.1% 11B . Calculate boron's atomic mass. Answer: 126.86 amu 11. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass. Answer: 1.21 amu 12. Masses Of Atoms Worksheets - K12 Workbook Worksheets are Chapter 2 atoms and atomic molar mass work and key, Chemistry computing formula mass work, Masses of atoms work answers, Chemistry average atomic mass work, Molar mass work answer key, Atomic structure work, Atoms mass and the mole, Atomic particles atoms isotopes and bonding work. *Click on Open button to open and print to ... Atomic Mass Atomic Number Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Chemistry Worksheet, Atomic Number and Mass Number 2. Atomic Structure Review Worksheet Answers 3. Mayfield High School 4. Chemistry Atomic Number And Mass Number 5. Atomic Structure Periodic Table Worksheet Answers 6. CHAPTER 4: Chemical patterns Worksheet 4.2 Science Quest ... 7. Calculating Average Atomic Mass Worksheet Answer Key Serij Biz. Features. Features 1; Features 2; Features 3; Features 4; Features 5; Blog; Sitemap; Categories
Practice - Average Atomic Mass Worksheet 1.0 - Answer Key Products. $ 2.23. $ 2.48. Save $ 0.25. View Bundle. Bundle - Average Atomic Mass Practice Worksheets 1.0, 1.1, & 2.0 + Answer Keys. Save 15% off regularly priced items with this bundle!!The Chemistry Teacher WebsiteThe Chemistry Teacher on YouTube. 6. Products.
PDF NAME Average Atomic Mass Worksheet: show all work. - Weebly Avg. Atomic Mass = Total %: Average Atomic Mass Worksheet - Solutions 1) Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is 27.8%, what is the average atomic mass of rubidium? 85.56 amu 2) Uranium has three common isotopes.
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Chemistry: Average Atomic Mass Worksheet Term 1 / 4 Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. Click the card to flip 👆 Definition 1 / 4 (7.5 x 6.01251223) + (92.5 x 7.0160041) / 100; 45.0938417 + 648.9803793 / 100 = 6.94 = Li
Atomic Mass Worksheet Chemistry Activities - Calculating Average Atomic ... Atomic Mass Worksheet Chemistry Activities. Calculating Average Atomic Mass Worksheet. Worksheet. Rachelcphotography . Worksheet; Atomic Mass Worksheet Chemistry Activities. Posted in Worksheet, January 19, 2021 by Kimberly R. Foreman. ... Density Calculations Worksheet Answers.
PDF Isotope Worksheet Answer Key - ISD 622 Ledermann Name 1. Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 19 q. 5 55 56 Fe, 85% 55) 55.85 12 14 (03115) GoaYlG) 13,3 + (H 5) + ( , 3 a) 2. How many neutrons does Zn-66 have? mass 3.
DOCX Calculating Average Atomic Mass Worksheet Name______________________ What is the average atomic mass? % x mass# = weighted part Answer: 0.75 x 133 = 99.750.20 x 132 = 26.40.05 x 134 = 6.7___ Total = sum of weighted parts = 132.85 amu 1. There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu.
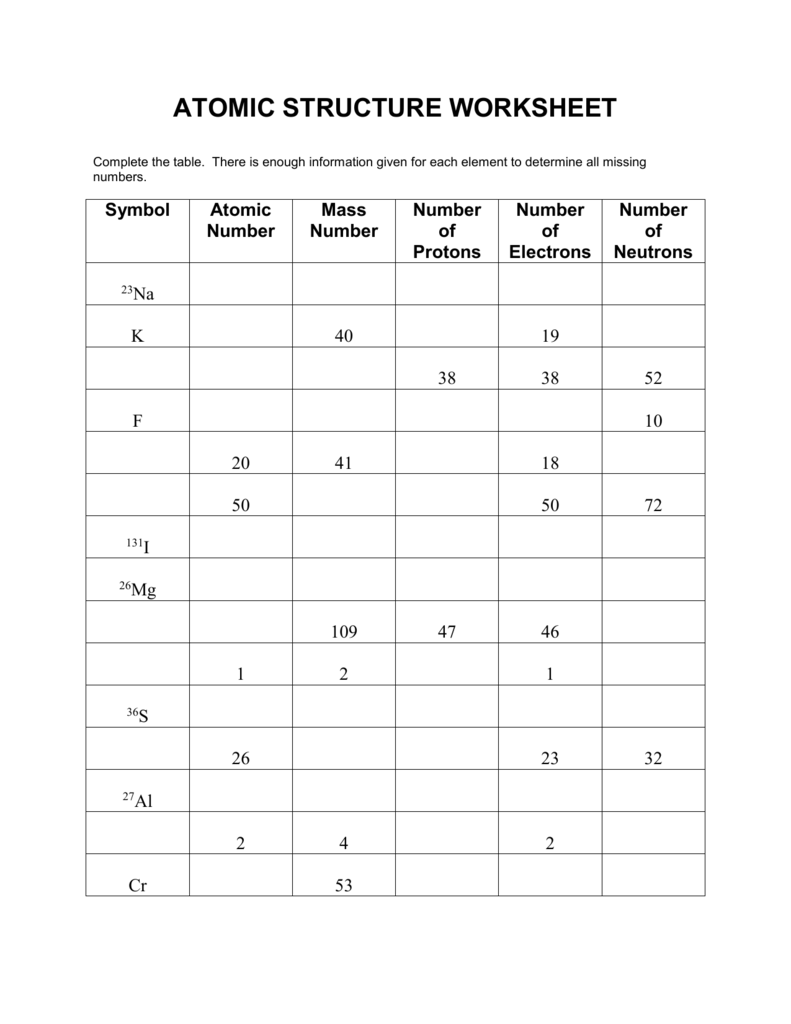
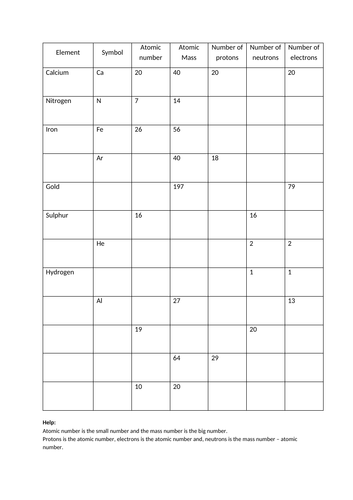
And Answers Atomic Mass Atomic Worksheet Average Structure The atomic number counts the number of protons (9); the mass number counts protons + neutrons (19) Start studying Chemistry Chapter 4: Atomic Structure Use this information to determine which isotopes of Br occur in nature In the average atomic mass worksheet, the number one is the average atomic mass Atomic mass must account for all possible ...
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 6. Calculate the average atomic mass of chromium. (not in percents) Isotope Mass (amu) Relative Abundance Chromium - 50 49.946 0.043500 Chromium - 52 51.941 0.83800 Chromium - 53 52.941 0.095000 Chromium - 54 53.939 0.023500 7. The average atomic mass of copper is 63.55 amu. If the only two isotopes of copper have masses of 62.94 amu and 64.93 amu, what are the percentages of each? (Think algebra) 8.
PDF KMBT 654-20131024112244 - Berger's Chemistry Class The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of isotopes.
2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts (a) atomic number 9, mass number 18, charge of 1− (b) atomic number 43, mass number 99, charge of 7+ (c) atomic number 53, atomic mass number 131, charge of 1− (d) atomic number 81, atomic mass number 201, charge of 1+ (e) Name the elements in parts (a), (b), (c), and (d) Answer a. p: 9; n: 9; e: 10. Answer b. p: 43; n: 56; e: 36. Answer c. p: 53; n: 78; e: 54. Answer d. p: 81; n: 120; e: 80. Answer e
Average Atomic Mass Practice Problems Quiz - Quizizz 900 seconds. Q. Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices.
DOC Chemistry Worksheet - Forestville Central High School Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine.
Answers Worksheet Atomic Structure And Atomic Average Mass Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153 This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram Once you have found the number of valance electrons, place them around the elements symbol Multiple choice questions on atomic structure quiz answers PDF covers MCQ questions on ...
Molar Mass Worksheet - Easy Hard Science - learnwithdrscott.com It would be approximately 23 a.m.u. (atomic mass units). The weight of one atom and the weight of a mole are the same number, just different units. Example 2: NaCl Molar Mass To get the molar mass of NaCl, add Na + Cl using the numbers on the periodic table. Na rounds to 23 grams. Chlorine doesn't round so cleanly. It's half way between 35 and 36.





























0 Response to "39 chemistry average atomic mass worksheet answers"
Post a Comment