38 chemistry unit 1 worksheet 6 answers
en.wikipedia.org › wiki › ChemistryChemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. wou.edu › chemistry › coursesChapter 6 – Quantities in Chemical Reactions – Chemistry Using formulas to indicate how many atoms of each element we have in a substance, we can relate the number of moles of molecules to the number of moles of atoms. For example, in 1 mol of ethanol (C 2 H 6 O), we can construct the following relationships (Table 6.1 “Molecular Relationships”): Table 6.1: Molecular Relationships
› reviews › Momentum-andMomentum and Collisions Review - with Answers #2 Use 1 kg as the mass of the carts (or any number you wish) and then set the expression for initial total momentum equal to the expression for the final total momentum: (1 kg)*(2) + (1 kg) *(-1) = (1 kg) *v + (1 kg) *v. Now solve for v using the proper algebraic steps. (2 kg•m/s) - (1 kg•m/s) = (2 kg) v. 1 kg•m/s = (2 kg)v (1 kg•m/s ...

Chemistry unit 1 worksheet 6 answers
› files › programsUnit Conversions Worksheet - Everett Community College 2.6 m3 x 102 cm x 102 cm x 102 cm x 1 mL = 2.6 x 106 mL ... Unit Conversions Worksheet Author: Moira O'Toole Subject: chemistry Created Date: 6/16/2011 12:59:51 PM ... › reviews › Work-and-EnergyWork and Energy Review - with Answers #1 - Physics Classroom FALSE - Watt is the standard metric unit of power; Joule is the standard metric unit of energy. c. TRUE - A N•m is equal to a Joule. d. TRUE - A kg•m 2 /s 2 is a mass unit times a speed squared unit, making it a kinetic energy unit and equivalent to a Joule. e. wou.edu › ch105-chapter-1-measurements-chemistryCH105: Chapter 1 – Measurements in Chemistry – Chemistry Thus, if we have 6.022 × 10 23 Oxygen atoms, we say we have 1 mol of Oxygen atoms. If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6 H 6) molecules, we have 0.5 × (6.022 × 10 23) C 6 H 6 molecules, or 3.011 × 10 23 C 6 H 6 molecules.
Chemistry unit 1 worksheet 6 answers. › indexPHSchool.com Retirement–Prentice Hall–Savvas Learning Company PHSchool.com was retired due to Adobe’s decision to stop supporting Flash in 2020. Please contact Savvas Learning Company for product support. wou.edu › ch105-chapter-1-measurements-chemistryCH105: Chapter 1 – Measurements in Chemistry – Chemistry Thus, if we have 6.022 × 10 23 Oxygen atoms, we say we have 1 mol of Oxygen atoms. If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6 H 6) molecules, we have 0.5 × (6.022 × 10 23) C 6 H 6 molecules, or 3.011 × 10 23 C 6 H 6 molecules. › reviews › Work-and-EnergyWork and Energy Review - with Answers #1 - Physics Classroom FALSE - Watt is the standard metric unit of power; Joule is the standard metric unit of energy. c. TRUE - A N•m is equal to a Joule. d. TRUE - A kg•m 2 /s 2 is a mass unit times a speed squared unit, making it a kinetic energy unit and equivalent to a Joule. e. › files › programsUnit Conversions Worksheet - Everett Community College 2.6 m3 x 102 cm x 102 cm x 102 cm x 1 mL = 2.6 x 106 mL ... Unit Conversions Worksheet Author: Moira O'Toole Subject: chemistry Created Date: 6/16/2011 12:59:51 PM ...













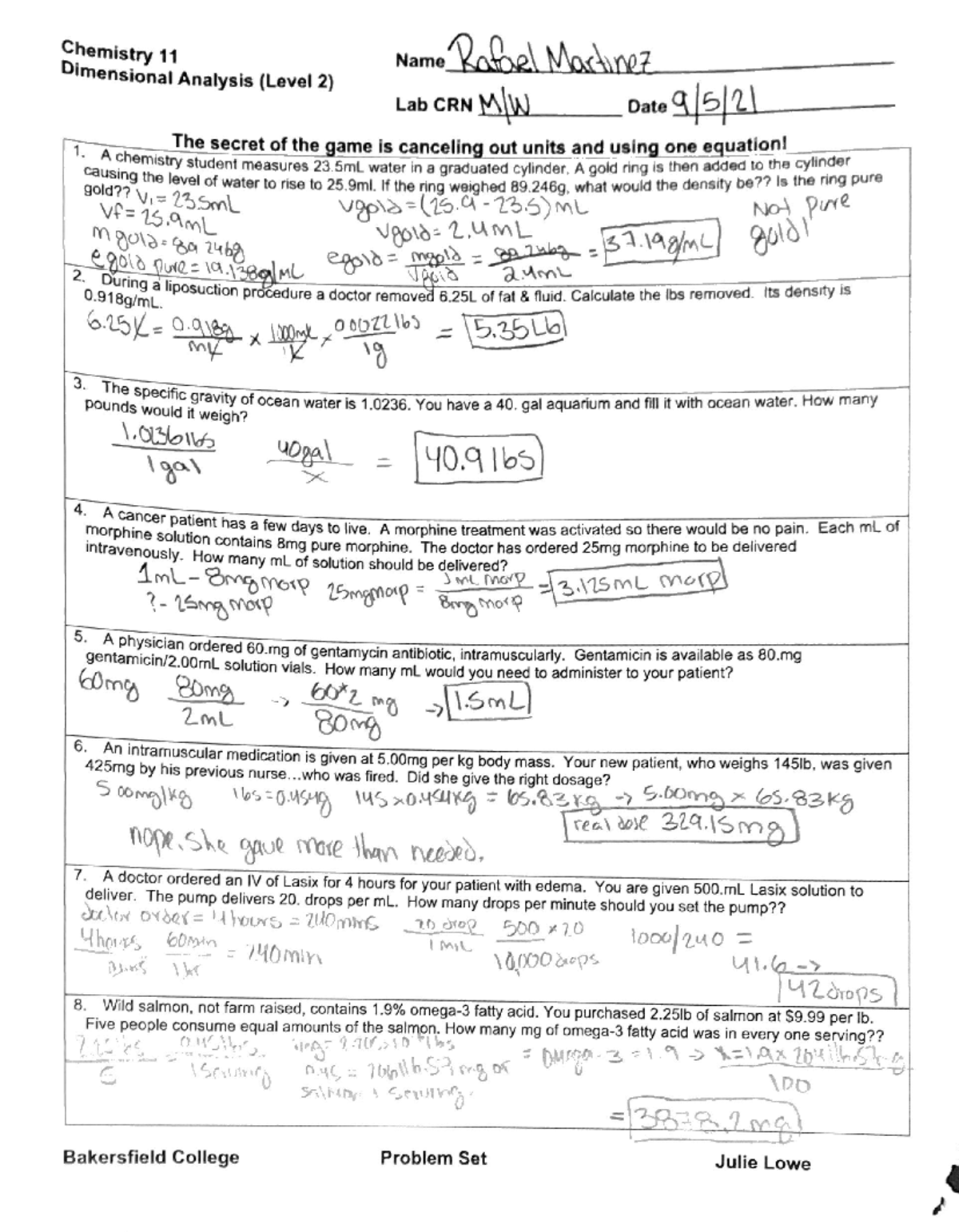













0 Response to "38 chemistry unit 1 worksheet 6 answers"
Post a Comment