43 significant figures worksheet chemistry
Significant Figures Questions - Practice Questions of Significant ... There are three significant figures in the first number, and four significant figures in the second. As a result, we only use three significant figures in our final answer: 76.4 180.4 = 13,782.56 = 13,800. The first number has four significant figures, whereas the second number only has three. PDF Significant Figures Practice Worksheet - Laney College Title: Significant Figures Practice Worksheet Author: Ian Guch Subject: Created Date: 6/16/2000 11:54:37 PM
Significant Figures Worksheet Chemistry - Edu Stiemars Significant Figures Worksheet Chemistry. The first three zeros are insignificant, but the zero between the sixes is, hence this number has four important figures. First thing, the foundations of algebra say to do the subtraction first, then the division. Similarly, 1.01 has three vital digits and two digits after the decimal point.
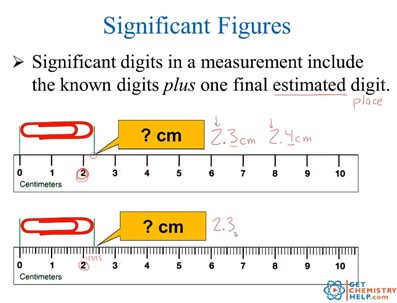
Significant figures worksheet chemistry
Significant Figures | Pathways to Chemistry Significant Figures 1. Matter and Measurement in Chemistry Matter The Scientific Method Measurement, Metric System, and SI Units Mass,... 2. Atoms, Ions, and Molecules Introduction to Elements and the Periodic Table The Periodic Table and its Design Common... 3. Chemical Reactions and Mass Balancing ... PDF Chemistry: Significant Digits - SMATCOE Chemistry: Significant Digits 1. Significant numbers are always measurements and thus should always be accompanied by the measurement's unit. For simplicity, units are not included in the following examples. 2. Any numbers (that are measurements) other than zero are significant. (Many times the zeros are also significant as you will see below.) PDF Significant figures worksheet answers - Stockbridge Valley Central School Cm 2) Re-write the quantity 827,000,000,000,000 picoseconds to show: a) 1 sig. fig. b) 2 sig. figs. c) 3 sig. figs. d) 4 sig. figs. e) 5 sig. figs.
Significant figures worksheet chemistry. Significant Digits - Elizabethtown Area School District contains three significant digits. 5. Any zeros to the left of a number but to the right of a decimal point are not significant. 921000. has six significant digits. 6. These zeros are present merely to indicate the presence of a decimal point (they are used as place holders), (these zeros are not part of the measurement). The number 0.00123 Significant Figures ID: 2248041 Language: English School subject: Chemistry Grade/level: 10-12 Age: 14-18 Main content: Significant Figure calculations Other contents: Add to my workbooks (4) Download file pdf Add to Google Classroom Add to Microsoft Teams PDF Worksheet 1: Significant Figures - Gwinnett County Public Schools Worksheet 1: Significant Figures . 1. Determine the number of significant figures in each of the following. a) 0.1890 . b) 1.380 . c) 10 . d) 110.0 . e) 1.02 x 10-4 . ... AP Chemistry: Review Unit N . Worksheet 2: Unit Conversions . Complete the following unit conversations, paying attention to significant figures. Significant Figures in Chemistry ~ ChemistryGod Rule 5. When a number is without the decimal point, all zeros at the end (after the last non-zero digit) may or may not be significant. Consider a number 1 400, It may have 2, 3, or 4 significant figures. It is not possible to estimate whether the number is certain up to ± 1, ± 10, or ± 100.
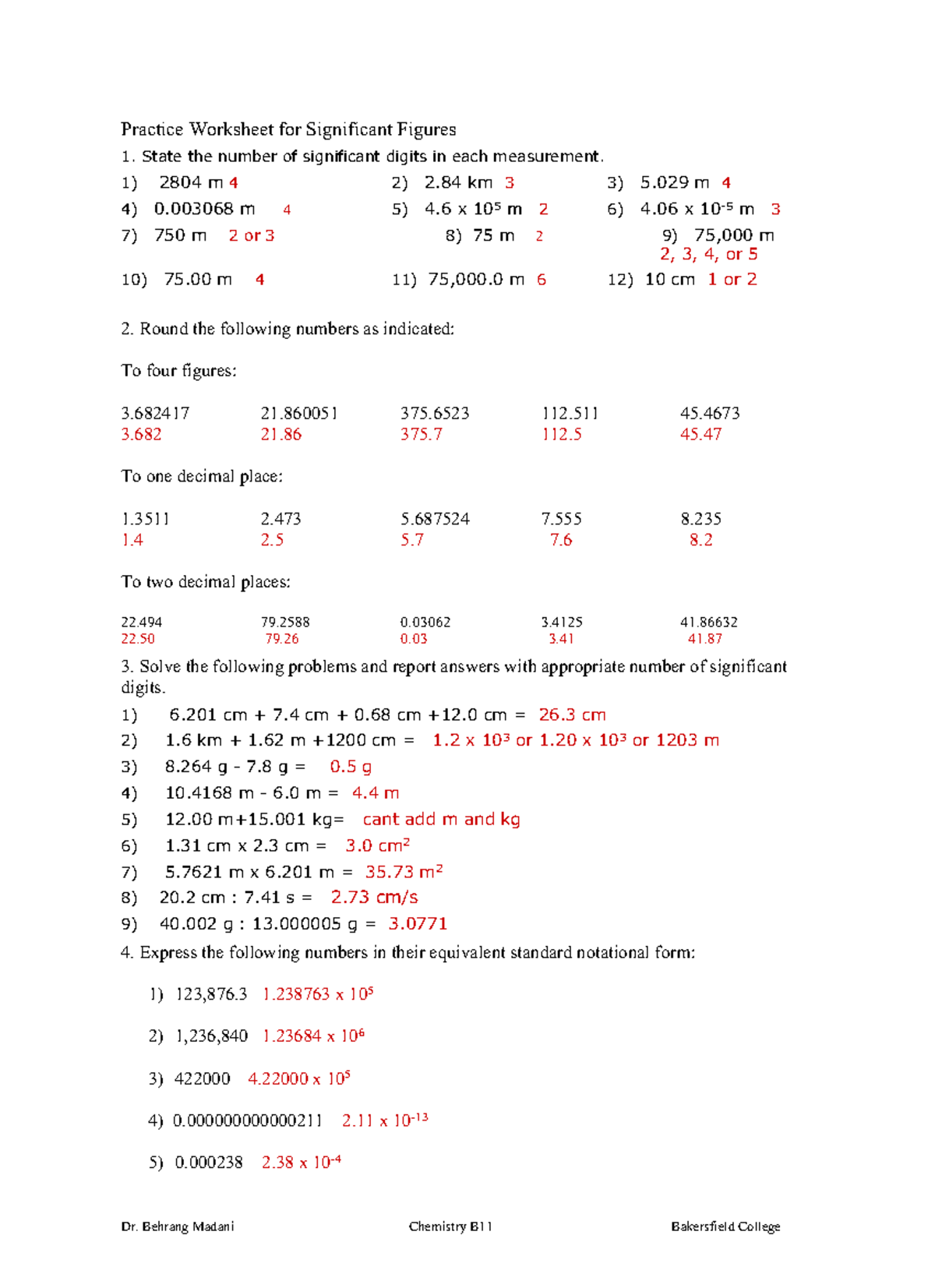
PDF Practice Worksheet for Significant Figures - Doolan's Chemistry Class Period:_____ Practice Worksheet for Significant Figures Name:_____ Identify the sums or differences of the following to the correct number of significant figures: 41) 8.41 X 1014 + 9.71 X 1012 = 8.51 E 14 42) 5.11 X 1023 - 4.2 X 1021 = 5.1 E 23 43) 8.2 X 1030 + 4.560 X 1023 = 8.2 E 30 44) 6.023 X 10-23 - 2.1 X 10-20 = -2.9 E -20 Express the product and the quotients of the following to the ... PDF Practice Worksheet for Significant Figures 1. State the number of significant digits in each measurement. 1) 2804 m2) 2.84 km3) 5.029 m 4) 0.003068 m5) 4.6 x 105m 6) 4.06 x 10-5m 7) 750 m8) 75 m 9) 75,000 m 10) 75.00 m 11) 75,000.0 m 12) 10 cm 2. Significant Figures | The Cavalcade o' Chemistry Accuracy vs. precision. Significant figures tell you how precise a measured value is. A measurement of "430 grams" is precise to the nearest ten grams, as indicated by significant figures. Precision is a measurement of how reproducible an answer is with some piece of equipment. If an instrument is precise, it will give you an answer that ... Significant Digits worksheet-2 - Chemistry: Significant Digits ... The number 0 has three significant digits. The reason that these zeros are not significant is that the measurement 0 grams is equal in magnitude to the measurement 1 milligrams. 1 has three significant digits, thus 0 must also have three significant digits. Any zeros to the right of a number and the right of a decimal point are significant.
PDF Significant Figures H 204 - Everett Community College Significant Figures H 204 1) Multiplication/ Division Rules: The number that has "the fewest number of Significant Figures" decides the number of significant figures in the final answer. Example: 2.711 x 6.3 = 17.0793 => 4 SF 2 SF 2 SF in the final answer (Choose the fewest SF) 2) Addition/ Subtraction Rules: DOC Practice Worksheet for Significant Figures Practice Worksheet for Significant Figures 1. State the number of significant digits in each measurement. 1) 2804 m 4 2) 2.84 km 3 3) 5.029 m 4 4) 0.003068 m 4 5) 4.6 x 105 m 2 6) 4.06 x 10-5 m 3 7) 750 m 2 or 3 8) 75 m Worksheet on Significant Figures | Significant Figures Practice Worksheets Significant Figures Examples with Answers Check different problems impose on Significant Figures and get a grip on every concept available in the Significant Figures. 1. Find the Number of a Significant Figure in Each of the Following (a) 8.4 (b) 173.6 m (c) 407 g (d) 4.68 m (e) 8.1165 kg (f) 0.054 km (g) 0.00343 l (h) 93.040 mg (i) 30.030300 g Significant Figures - Introductory Chemistry - 1st Canadian Edition Therefore, we limit our final answer to three significant figures: 76.4 × 180.4 = 13,782.56 = 13,800. The first number has four significant figures, while the second number has three significant figures. Therefore we limit our final answer to three significant figures: 934.9 ÷ 0.00455 = 205,472.5275… = 205,000.
Significant Figures Worksheet - SIGNIFICANT FIGURES There are ... - StuDocu General Chemistry I (CHM 160) Uploaded by Trenton Barber Academic year 2018/2019 Helpful? Any zeros between two significant digits are significant. A final zero or trailing zeros in the decimal portion ONLY are significant. 3 x 10 9 250 780,000,
PDF Chem100 Worksheet 1a Scientific notation, significant figures Chem100 Worksheet 1a Scientific notation, significant figures Write each number in scientific notation 1. 538.35 2. 1750042 3. 0.00000000045 4. 21.3 Convert these numbers to decimal format. 5. 8.56 x 104 6. 78.4 x 102 7. 5.020 x 105 8. 1.39 x 10-6 How many significant figures are in each of the following numbers? 8. 22.405 __________
PDF Significant Figures Practice Worksheet - Everett Community College How many significant figures do the following numbers have? 1) 956 _____ 2) 2.1390 _____ 3) 4390 _____ 4) 0.500 _____ 5) 500 _____ 6) 5.9 x 104_____ 7) 0.40001 _____ 8) 1.7 x 10-3_____ 9) 650. 10) 4.150 x 10-4_____ 11) 3670000 _____ 12) 0.0000620 _____ 13) 96 _____
PDF Significant Figures Worksheet - Ms. Pasta's Classes Significant Figures Worksheet Key 1. Indicate how many significant figures there are in each of the following measured values. 246.32 5 sig figs 107.854 6 sig figs 100.3 4 sig figs 0.678 3 sig figs 1.008 4 sig figs 0.00340 3 sig figs 14.600 5 sig figs 0.0001 1 sig fig 700000 1 sig fig 350.670 6 sig figs
PDF SUPPORT Sheet Standard form and significant figures c 809972 to three significant figures d 06.345 to three significant figures e 7840 to three significant figures f 0.007319 to three significant figures 3 Carry out the following calculations expressing the numbers in standard form to the degree of accuracy indicated: (4 marks) a (4.567 105) (2.13 10−3) to three significant figures b 3(1.567 ...
Significant Figures Quiz : ChemQuiz.net Significant Figures Quiz : ChemQuiz.net Significant Figures Quiz This online quiz is intended to give you extra practice in counting significant figures ("sig figs") in decimal and scientific notation as well as simple arithmetic problems. Select your preferences below and click 'Start' to give it a try!
Significant figures worksheet with answers pdf chemistry In this course, students will study an overview of various branches of chemistry as well as the basic themes of general inorganic chemistry . 2. Zero digits that occur between nonzero digits are significant . 202 contains three significant figures ⎫ In these examples, the zeros 450.5 contains four significant figures ⎬ are part of a ...
PDF Pottsgrove School District / Pottsgrove School District Homepage SIGNIFICANT FIGURES Name A measurement can only be as accurate and precise as the instrument that produced it. scientist must be able to express the accuracy of a number, not just its numerical value. e can determine the acçuracy of a number by the number of significant figures it contains. 1) All digits 1-9 inclusive are significant.
Significant Figures Worksheet - Easy Hard Science - learnwithdrscott.com The Significant Figures Worksheet covers sig figs, as they say, a topic found in chemistry classes that doesn't exactly look like chemistry. In Plain English, significant figures are used for rounding numbers. Rounding is a simple concept we usually learn in elementary school. We all know how to round: 0-4 round down, and 5-9 rounds up.
Significant figures | The Cavalcade o' Chemistry Updated 11/20/21. Lots and lots of significant figures! Significant figures practice worksheet: You'll be significantly figuring in no time!; Significant figures calculations sheet: Do calculations using the magic of significant figures!; Significant Figures I: Practice finding how many significant figures a measured value has. Also helps you to figure out what significance you get from sig ...
Worksheet 1 - Scientific notation/ significant figures Worksheet 1 Scientific Notation/Significant Figures 1. Convert each of the following into scientific notation. 2. Determine the number of significant figures in each of the following: 3. Convert each into decimal form. 4. Calculate the following. Give the answer in correct scientific notation. a) 4.53 x 105 b) 1913.0 + 2.2 x 106 - 4.6 x 103
PDF Significant figures worksheet answers - Stockbridge Valley Central School Cm 2) Re-write the quantity 827,000,000,000,000 picoseconds to show: a) 1 sig. fig. b) 2 sig. figs. c) 3 sig. figs. d) 4 sig. figs. e) 5 sig. figs.
PDF Chemistry: Significant Digits - SMATCOE Chemistry: Significant Digits 1. Significant numbers are always measurements and thus should always be accompanied by the measurement's unit. For simplicity, units are not included in the following examples. 2. Any numbers (that are measurements) other than zero are significant. (Many times the zeros are also significant as you will see below.)
Significant Figures | Pathways to Chemistry Significant Figures 1. Matter and Measurement in Chemistry Matter The Scientific Method Measurement, Metric System, and SI Units Mass,... 2. Atoms, Ions, and Molecules Introduction to Elements and the Periodic Table The Periodic Table and its Design Common... 3. Chemical Reactions and Mass Balancing ...

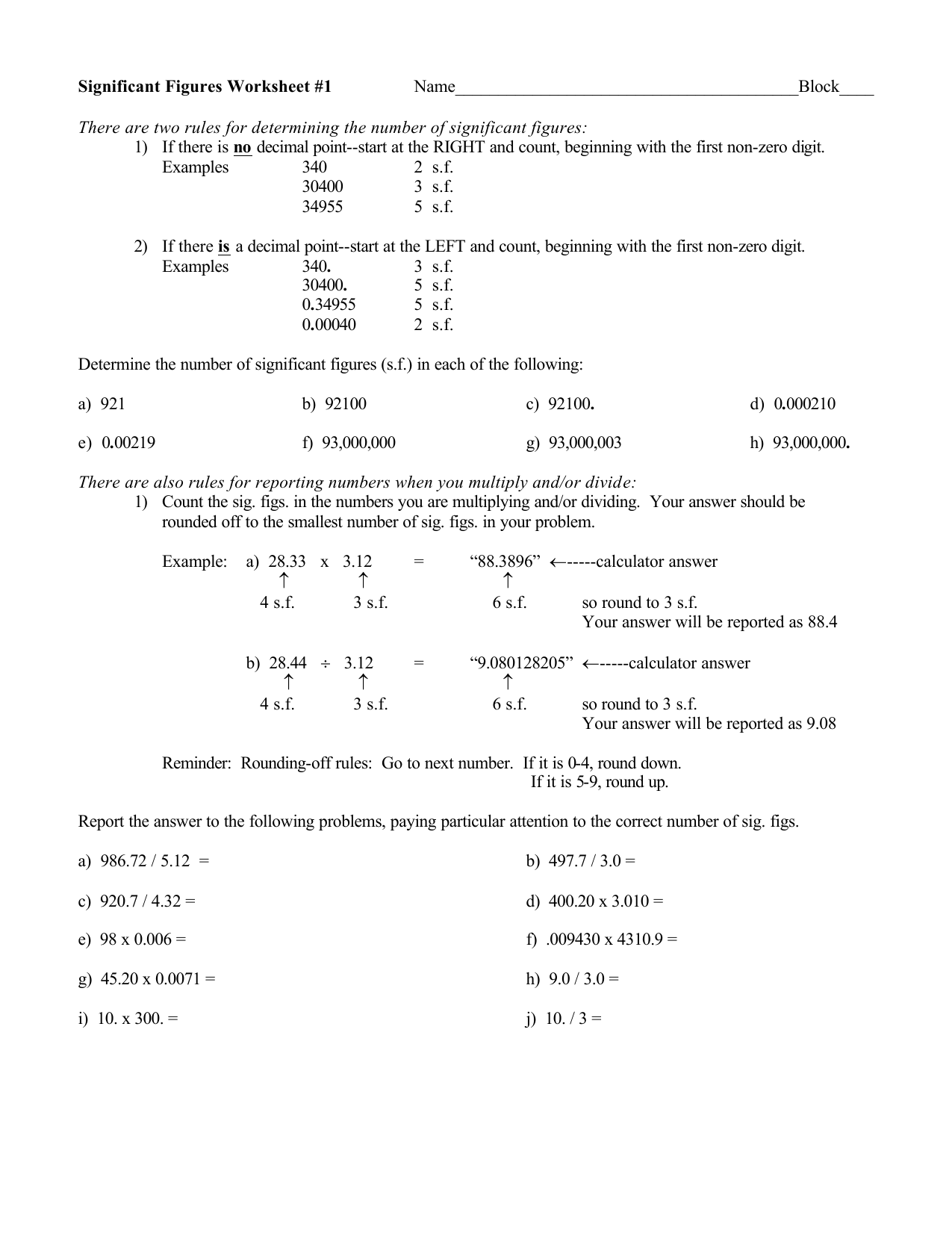









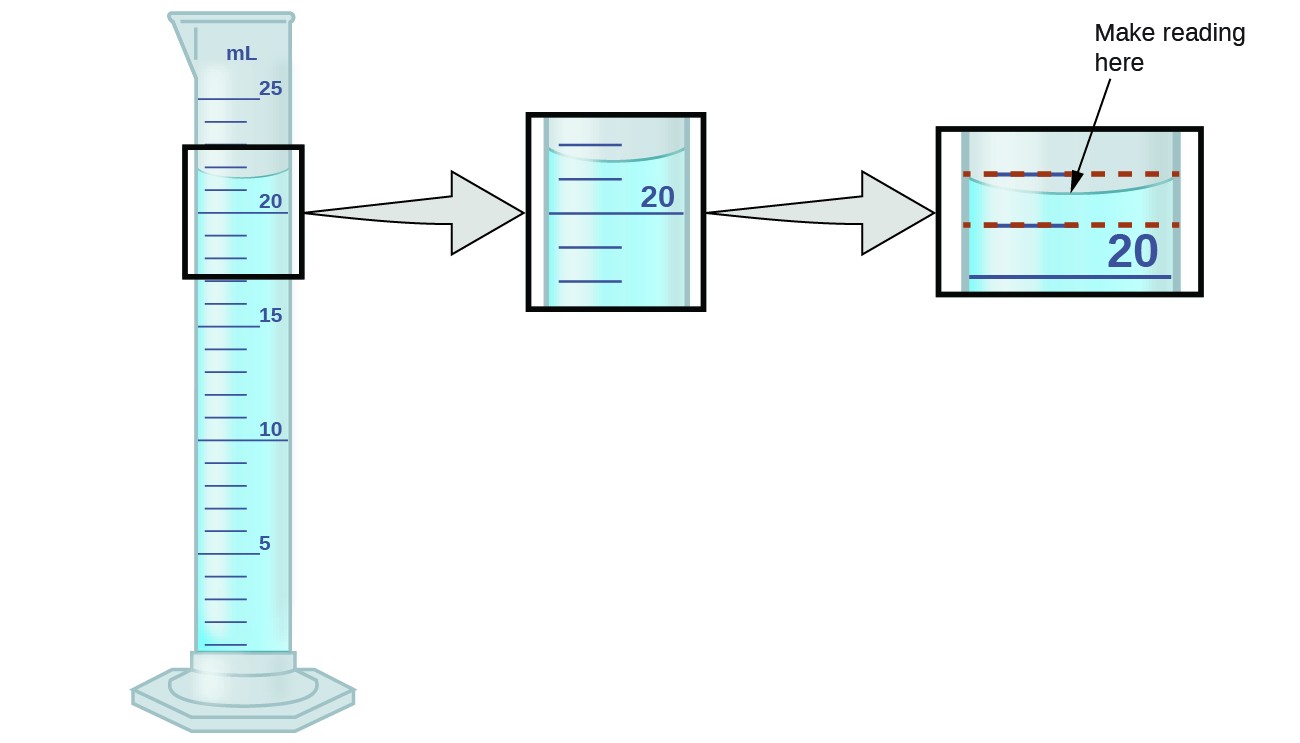














0 Response to "43 significant figures worksheet chemistry"
Post a Comment