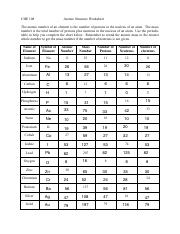
45 atomic mass and atomic number worksheet
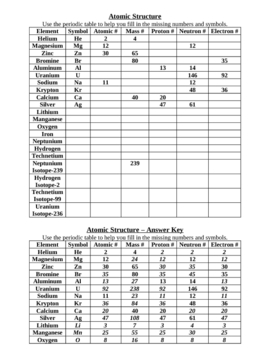
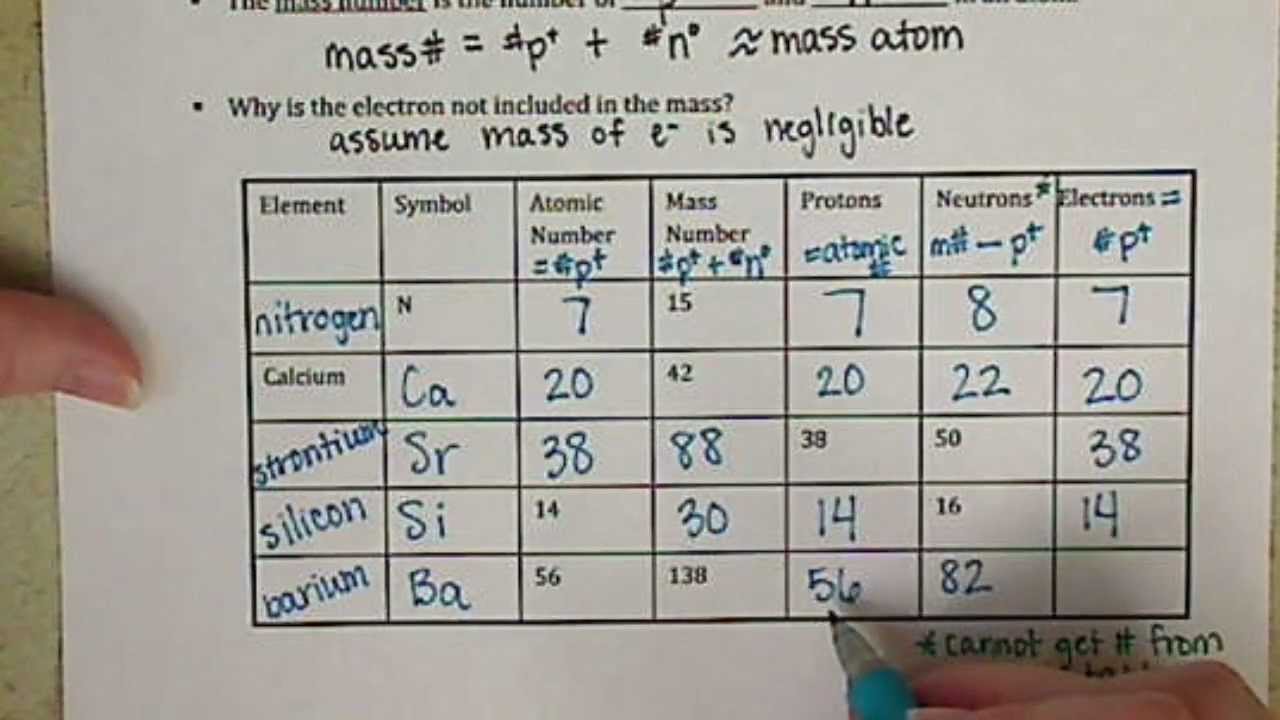
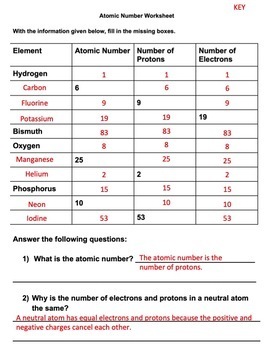
Chemistry of Matter - Science Spot Element Atomic Number Atomic Mass Protons Neutrons Electrons Li 3 4 3 P 15 16 15 Cl 17 18 17 Ni 59 28 28 K 19 19 20 Ag 108 47 47 H 1 0 1 Si 14 28 14 W 17 174 17 Ne 10 20 10 NOTE: The number protons and electrons is equal to the atomic number. To find neutrons, subtract the number of protons from the atomic mass. To find the atomic mass, add the number of protons … Atomic number - Wikipedia The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom, the sum of the atomic number Z and the neutron number N gives the atom's atomic mass number A.
Convert moles H2O to grams - Conversion of Measurement Units To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage ...

Atomic mass and atomic number worksheet
The change in mass when magnesium burns | Experiment | RSC ... To find the formula of magnesium oxide, students will need the mass of the magnesium and the mass of the oxygen. They will also require the relative atomic masses. Magnesium is 24 and oxygen is 16. They should divide mass by the atomic mass for each element. The gives the number of moles of each. Isotopes & Relative Atomic Mass (solutions, examples, videos) You put the atomic number, mass number, and net charge around the chemical element symbol. Isotope notation is particularly important in nuclear chemistry, because if you’re doing fission, fusion, alpha decay, beta decay, positron emission, or electron capture, you want to be able to tell how many neutrons and protons are in the nucleus. Quiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element?
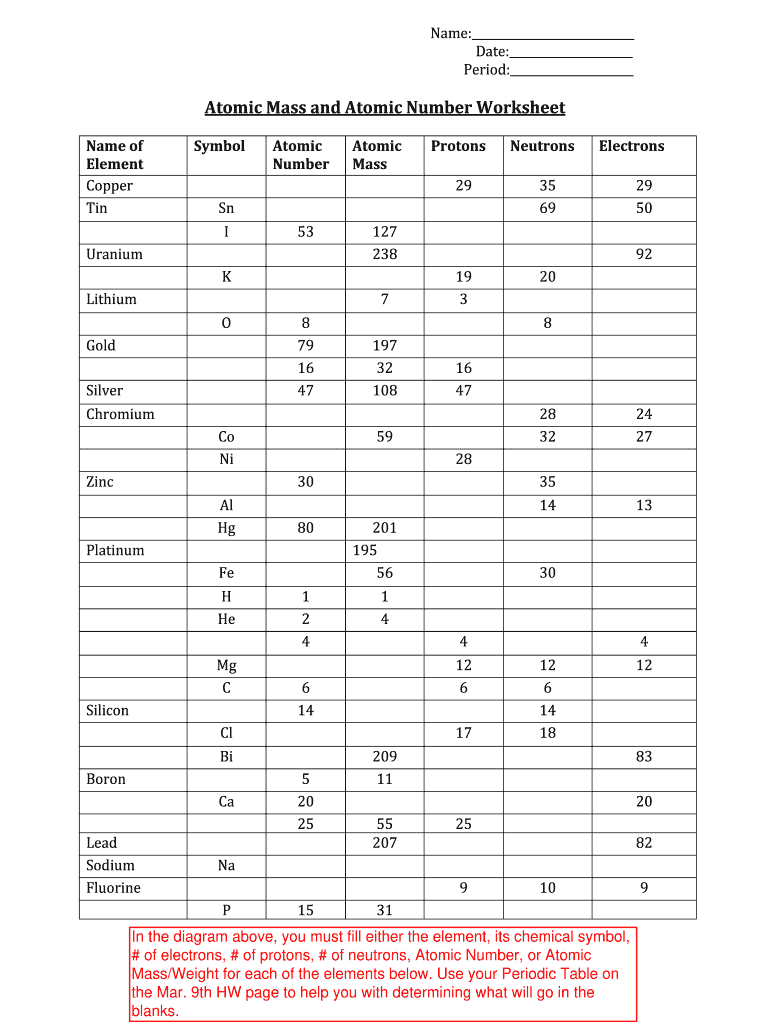
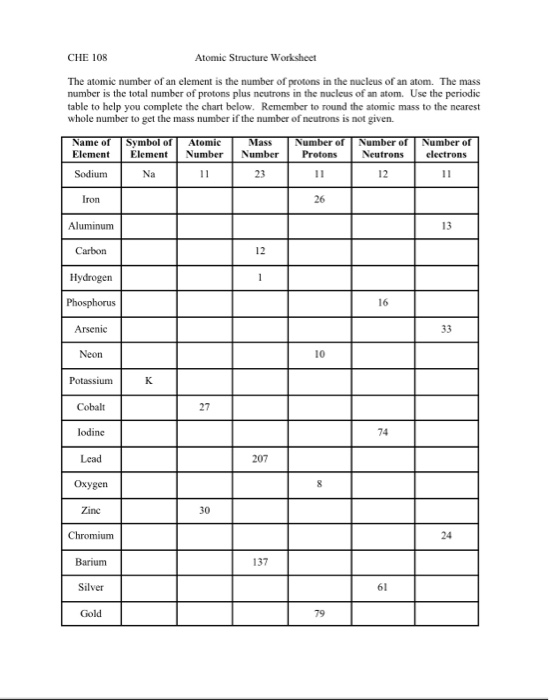
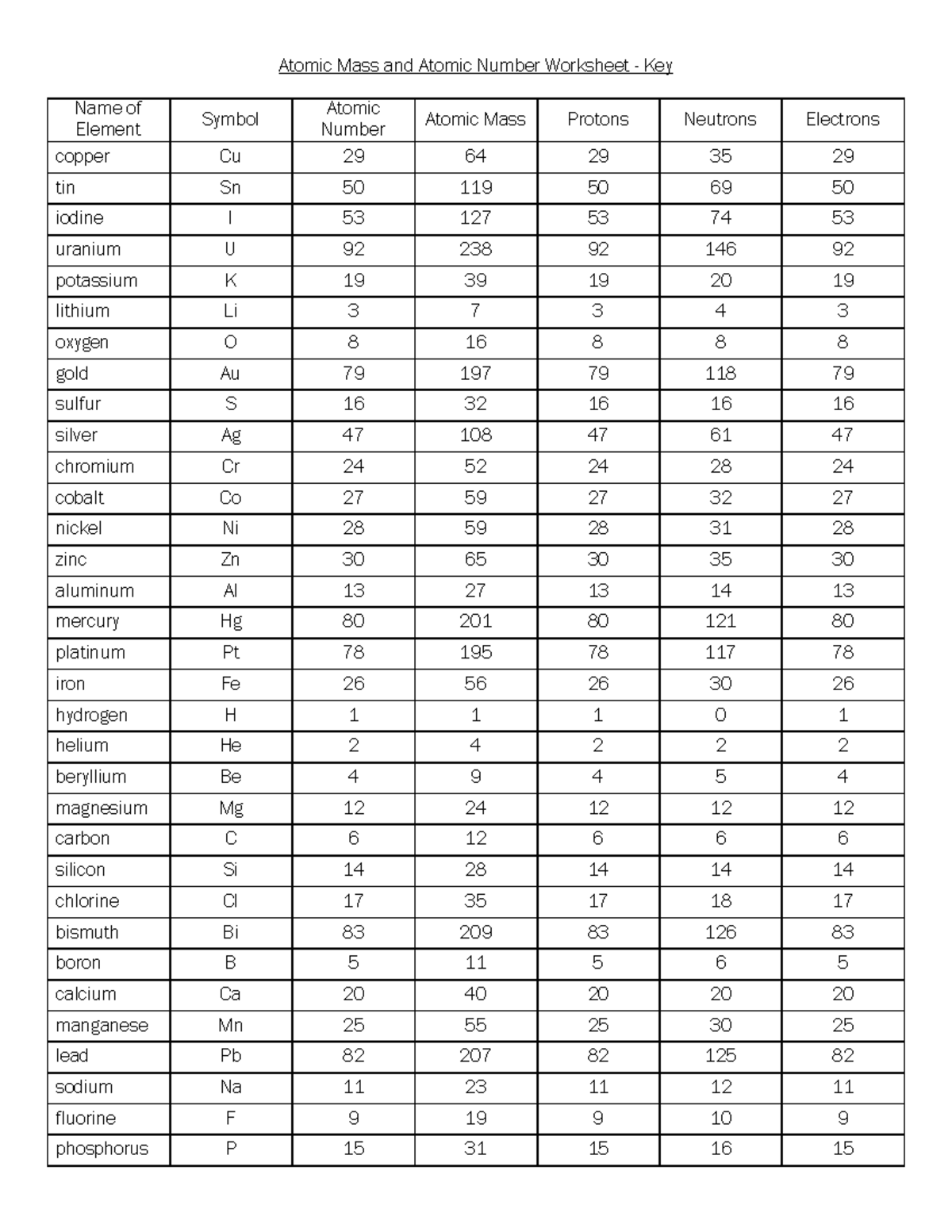
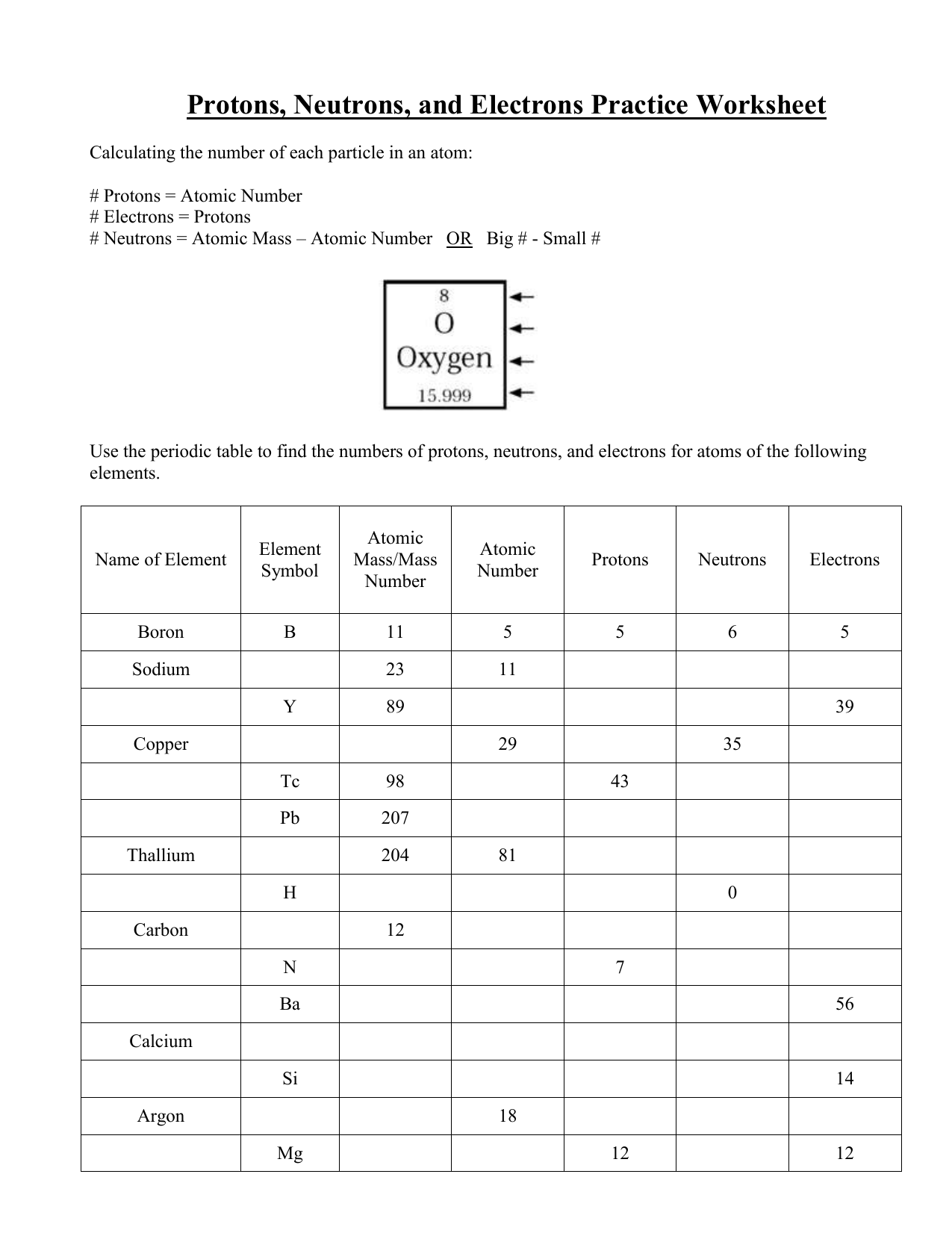
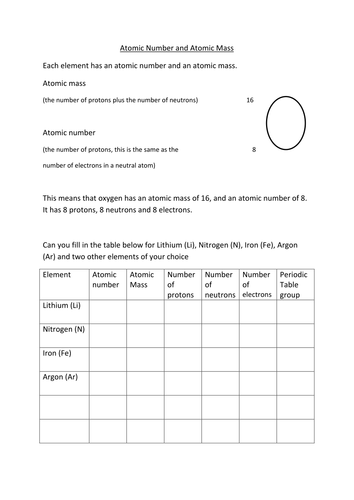
Atomic mass and atomic number worksheet. Atomic Structure Worksheet - WILLAMETTE LEADERSHIP … 9. What is the atomic number of the atom in the diagram above? 10. What is the atomic mass/mass number of the atom in the diagram above? 11. How many protons are in the nucleus of an atom with an atomic number of 15? 12. How many electrons are in the nucleus of an atom with an atomic number of 20? 13. How many neutrons are in the nucleus of an ... Atoms, Molecules, and Compounds On the periodic table, the atomic number is usually given as the whole number above the symbol for the element (see Fig. 2.13). For example, hydrogen (H) has an atomic number of one (1). This means a hydrogen atom has one proton. If a hydrogen atom is neutral, it must also have one electron. An oxygen atom (O) has an atomic number of eight (8). This means a neutral oxygen … Chemistry Simulations | CK-12 Foundation Atomic Mass Unit. Atomic Number. Bohr's Atomic Model. Calculating Atomic Mass. Electromagnetic Spectrum. Gold Foil Experiment . Isotope. Mass Number. Quantization of Energy. Spectral Lines of Hydrogen. Wavelength and Frequency Calculations. Chemical Bonding. Hydrogen Bonding. Polar Molecules. Van der Waals Forces. The Mole. Avogadro's Number. … Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 sulfur S 16 32 16 16 16 silver Ag 47 108 47 61 …
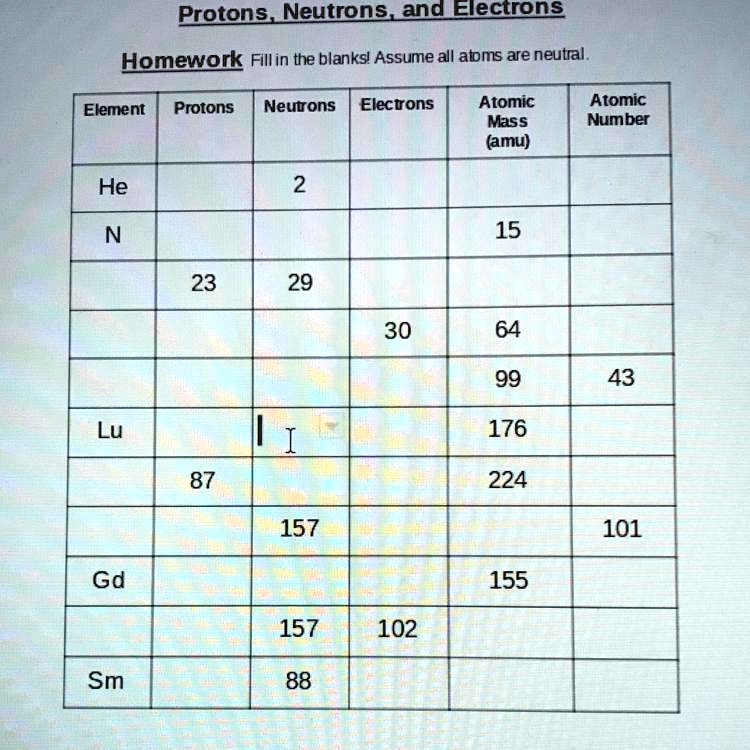
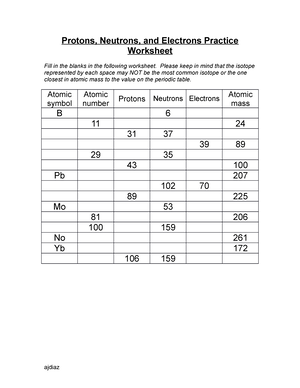
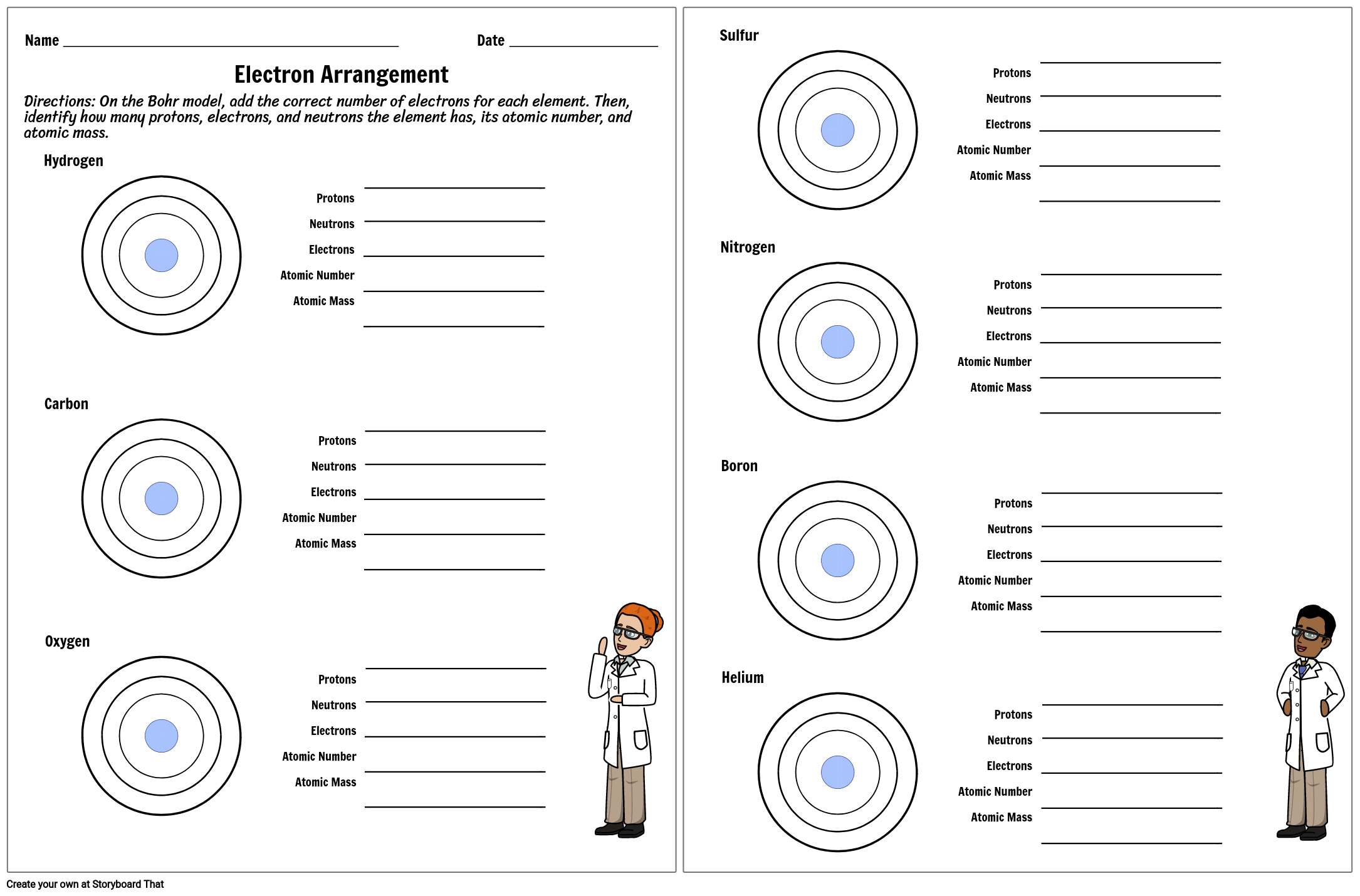
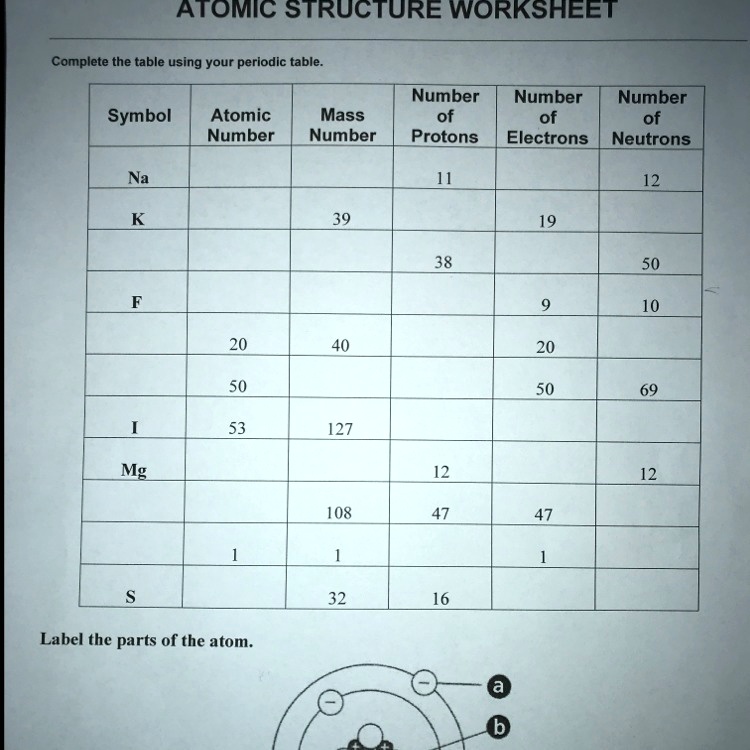
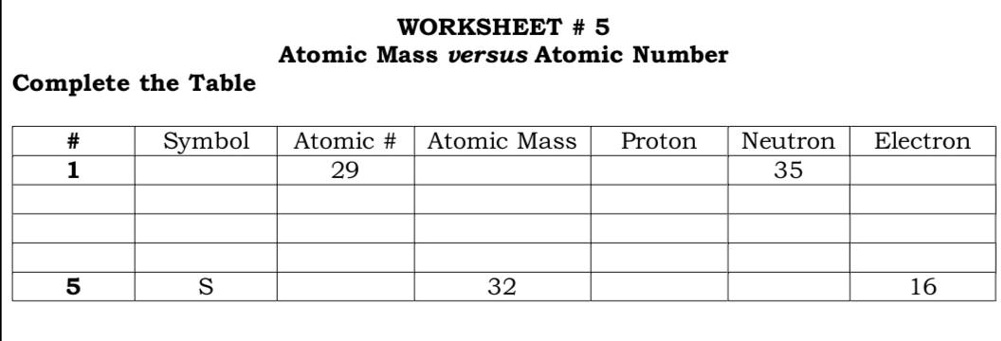
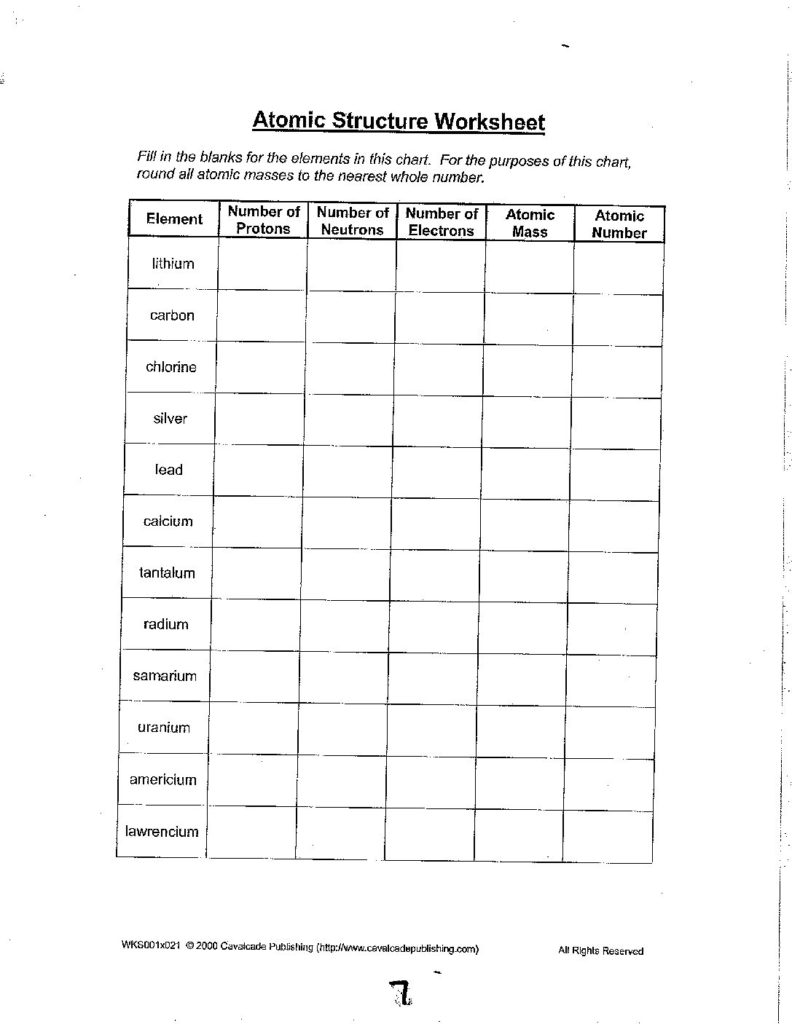
Atomic Structure Worksheet - Washoe County School District Which particles account for the mass of the atom? (Atomic mass or mass number) and. 8.Com lete the followin table. Symbol Atomic Number Number of Protons Number of Neutrons Number of Electrons Mass 9 The atomic number is the number of in one atom of an element. It is also the number of in a neutral atom of that element. The atomic number gives ... Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In Atomic Neutrons Electrons Atomic Charge Protons mass Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 +2 34 79 0 24 21 10 9 0 41 35 93 P 15 -3 Rb 85 +1 46 106 0 76 114 72 19 39 0 Mo 36 96 106 106 265 87 223 0 Hg 78 54 131 0 . Protons, Neutrons, and Electrons … KM 654e-20150109102424 - Columbia Public Schools How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table. No ...
Quiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? Isotopes & Relative Atomic Mass (solutions, examples, videos) You put the atomic number, mass number, and net charge around the chemical element symbol. Isotope notation is particularly important in nuclear chemistry, because if you’re doing fission, fusion, alpha decay, beta decay, positron emission, or electron capture, you want to be able to tell how many neutrons and protons are in the nucleus. The change in mass when magnesium burns | Experiment | RSC ... To find the formula of magnesium oxide, students will need the mass of the magnesium and the mass of the oxygen. They will also require the relative atomic masses. Magnesium is 24 and oxygen is 16. They should divide mass by the atomic mass for each element. The gives the number of moles of each.















:max_bytes(150000):strip_icc()/PeriodicTable-56a12c983df78cf772682271.png)










0 Response to "45 atomic mass and atomic number worksheet"
Post a Comment