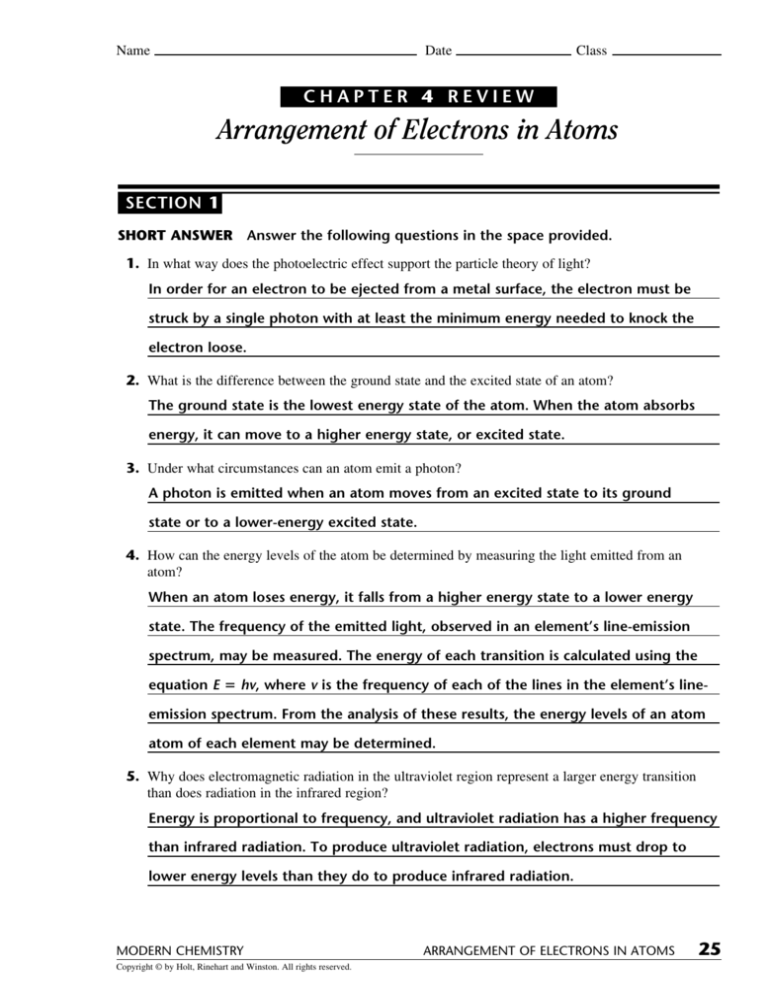
45 worksheet electrons in atoms
Chemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its subject, chemistry occupies an … Science for Kids: The Atom - Ducksters Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons. Atoms fit together with other atoms to make up matter. It takes a lot of atoms to make up anything. There are so many atoms in a single human body we won't even try to write the number here. Suffice it to say that the number …
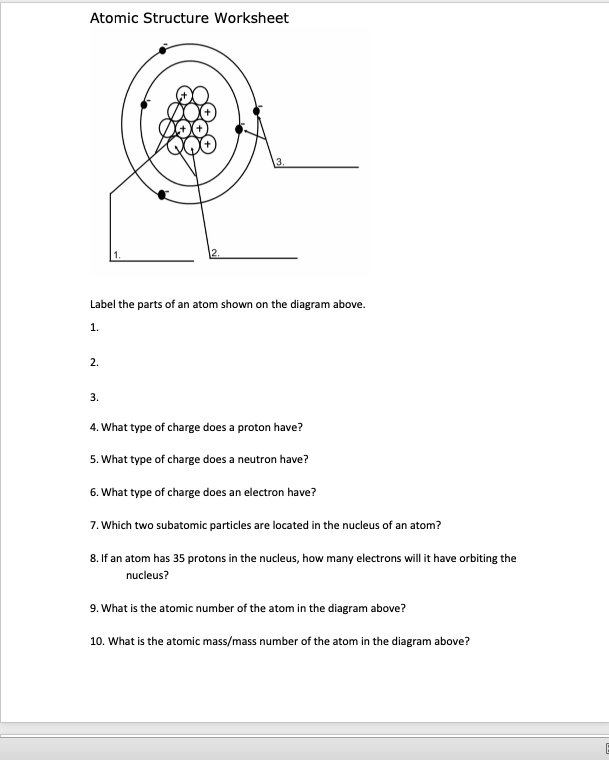
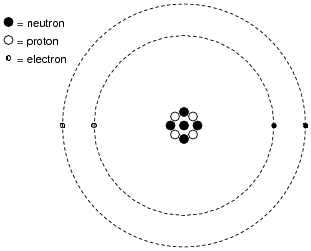
Atom Worksheets Atom is the most basic unit of matter. It has a dense nucleus with a cloud of negatively charged electrons surrounding it. Here are the parts of an Atom: Electron; it is a subatomic particle with a negative electrical charge. The mass of an electron is so small that it is generally not even considered. Nucleus; the dense center of an atom ...

Worksheet electrons in atoms
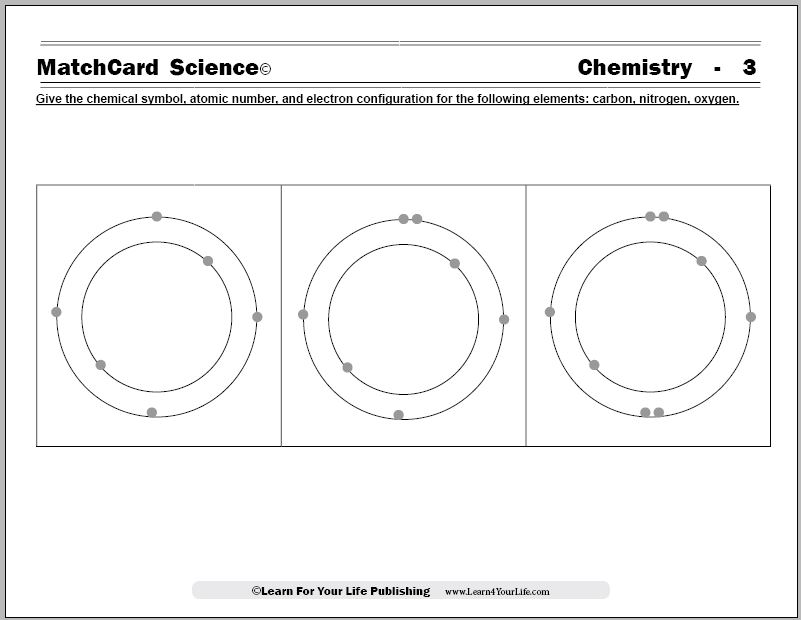
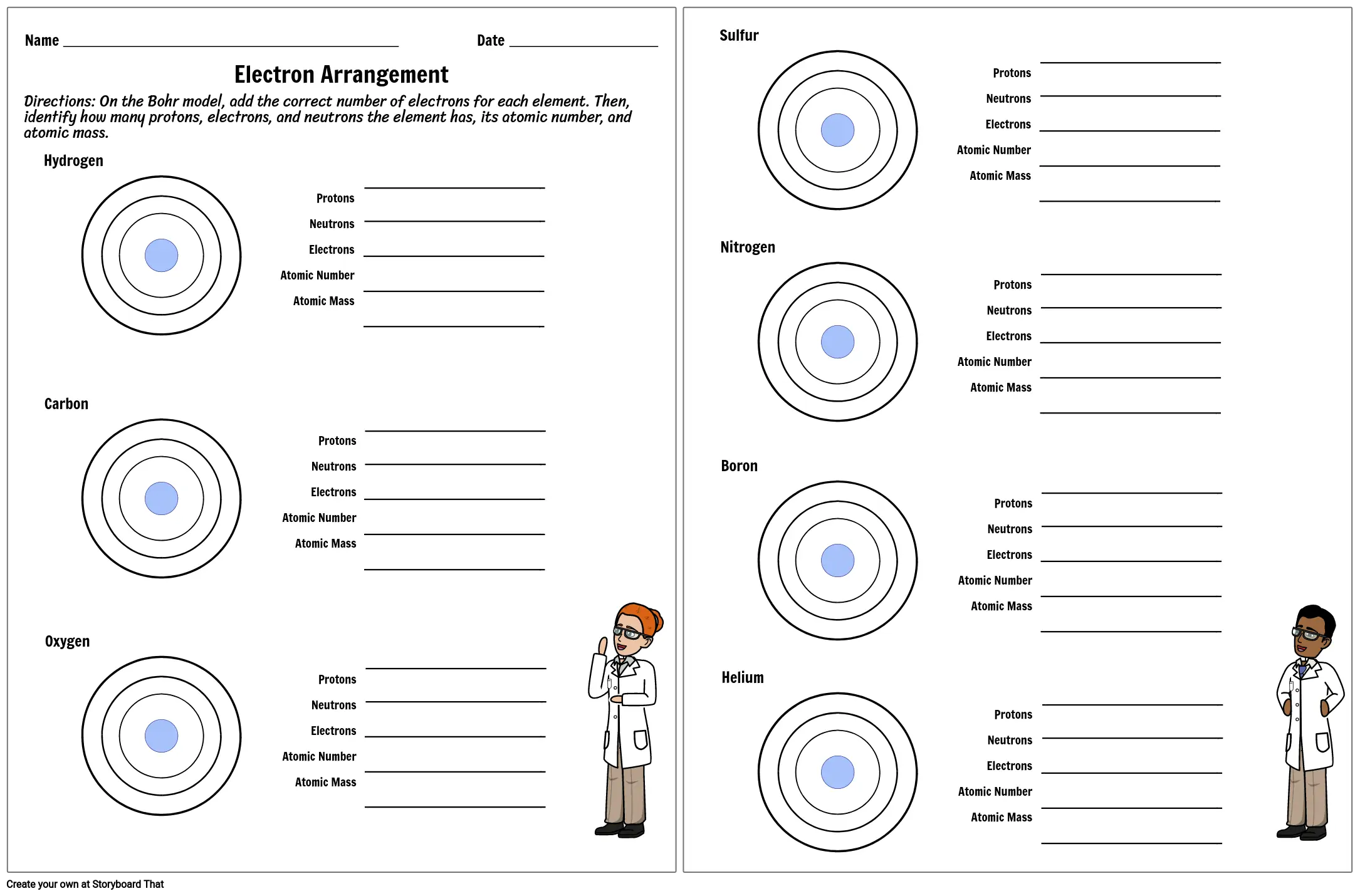
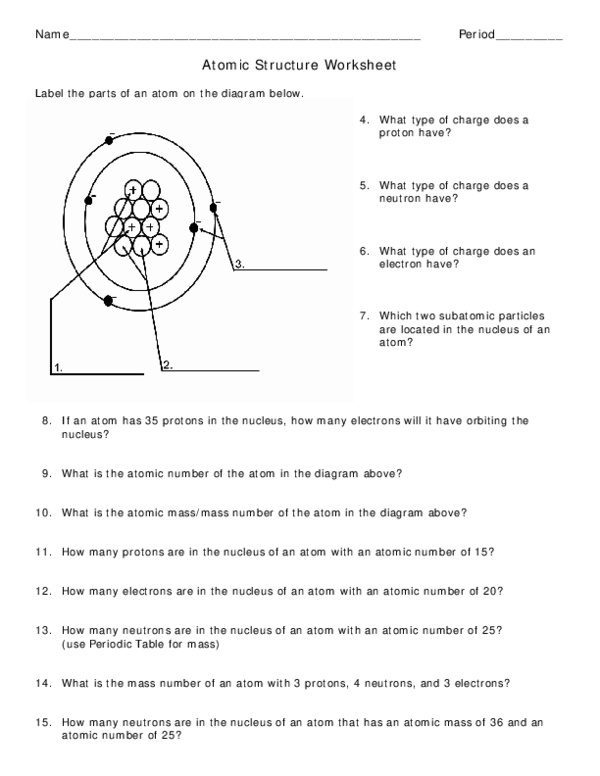
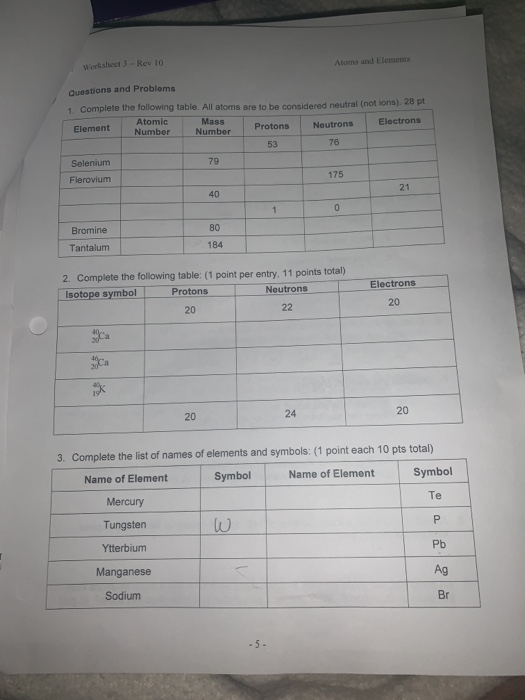
Protons, Neutrons, and Electrons Practice Worksheet - SMATCOE Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element Element Symbol Mass Number Atomic Number Protons Neutrons Electrons Boron B 11 5 5 6 5 Sodium 24 11 Y 89 39 Copper 29 35 Tc 98 43 Pb 207 Thallium 204 81 H 0 C arbon 12 N 7 Ba 56 Calcium Si 14 A rgon 18 Mg 12 12 . Oxygen 15.999 . … PHSchool.com Retirement–Prentice Hall–Savvas Learning … PHSchool.com was retired due to Adobe’s decision to stop supporting Flash in 2020. Please contact Savvas Learning Company for product support. Atoms, Molecules, and Compounds Parts of Atoms. The particles that make up an atom are called subatomic particles (sub- means “smaller size”).These particles are the. proton (p +), which is positively (+) charged;; electron (e –), which is negatively (–) charged; and; neutron (n 0), which has no charge, it is neutral (0).; Protons and neutrons occupy the nucleus, or center, of the atom.. Electrons exist in regions ...
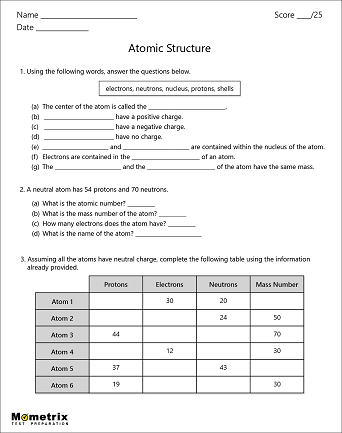
Worksheet electrons in atoms. Oxygen - Element information, properties and uses | Periodic Table The number of atoms of the element per 1 million atoms of the Earth’s crust. Recycling rate. The percentage of a commodity which is recycled. A higher recycling rate may reduce risk to supply. Substitutability. The availability of suitable substitutes for a given commodity. High = substitution not possible or very difficult. Atomic Structure Worksheet - Basic Electricity - All About Circuits Most helium atoms contain 2 neutrons, but some may contain more or less than 2. Each atom of aluminum is guaranteed to contain 13 protons. Unless the atom is electrically charged, it will contain 13 electrons as well to balance the charge of the protons. Most aluminum atoms contain 14 neutrons, but some may contain more or less than 14. Chemistry Help - Wyzant Lessons Polyatomic ions are ions with many atoms; they contain more than one element. They are charged just like regular . ions; for example, an ion looks like this: Cl –, while a polyatomic ion looks like this: ClO 3 –. While the chloride ion only contains one element – chlorine – the chlorate ion contains . both chlorine and oxygen. Read this ... Atomic Neutrons Electrons Atomic Charge Protons mass Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 +2 34 79 0 24 21 10 9 0 41 35 93 P 15 -3 Rb 85 +1 46 106 0 76 114 72 19 39 0 Mo 36 96 106 106 265 87 223 0 Hg 78 54 131 0 . Protons, Neutrons, and Electrons …
Atoms, Molecules, and Compounds Parts of Atoms. The particles that make up an atom are called subatomic particles (sub- means “smaller size”).These particles are the. proton (p +), which is positively (+) charged;; electron (e –), which is negatively (–) charged; and; neutron (n 0), which has no charge, it is neutral (0).; Protons and neutrons occupy the nucleus, or center, of the atom.. Electrons exist in regions ... PHSchool.com Retirement–Prentice Hall–Savvas Learning … PHSchool.com was retired due to Adobe’s decision to stop supporting Flash in 2020. Please contact Savvas Learning Company for product support. Protons, Neutrons, and Electrons Practice Worksheet - SMATCOE Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element Element Symbol Mass Number Atomic Number Protons Neutrons Electrons Boron B 11 5 5 6 5 Sodium 24 11 Y 89 39 Copper 29 35 Tc 98 43 Pb 207 Thallium 204 81 H 0 C arbon 12 N 7 Ba 56 Calcium Si 14 A rgon 18 Mg 12 12 . Oxygen 15.999 . …




























0 Response to "45 worksheet electrons in atoms"
Post a Comment