42 mole worksheet #1
Physics Simulation: Vector Addition - Physics Classroom This collection of interactive simulations allow learners of Physics to explore core physics concepts by altering variables and observing the results. This section contains more than 70 simulations and the numbers continue to grow. Convert moles H2O to grams - Conversion of Measurement Units The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles H2O, or 18.01528 grams. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between moles H2O and gram. Type in your own numbers in the form to convert the units! ››
CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack XOn+1 y– per‐ + stem + ‐ate SO 5 2– persulfate Xy– stem + ‐ide (the monatomic ion) S2– sulfide Note that in some cases, the ‐ate form has three oxygen atoms, and in some cases four oxygen atoms. C. Naming Ionic Compounds Writing Formulas of Ionic Compounds 1.
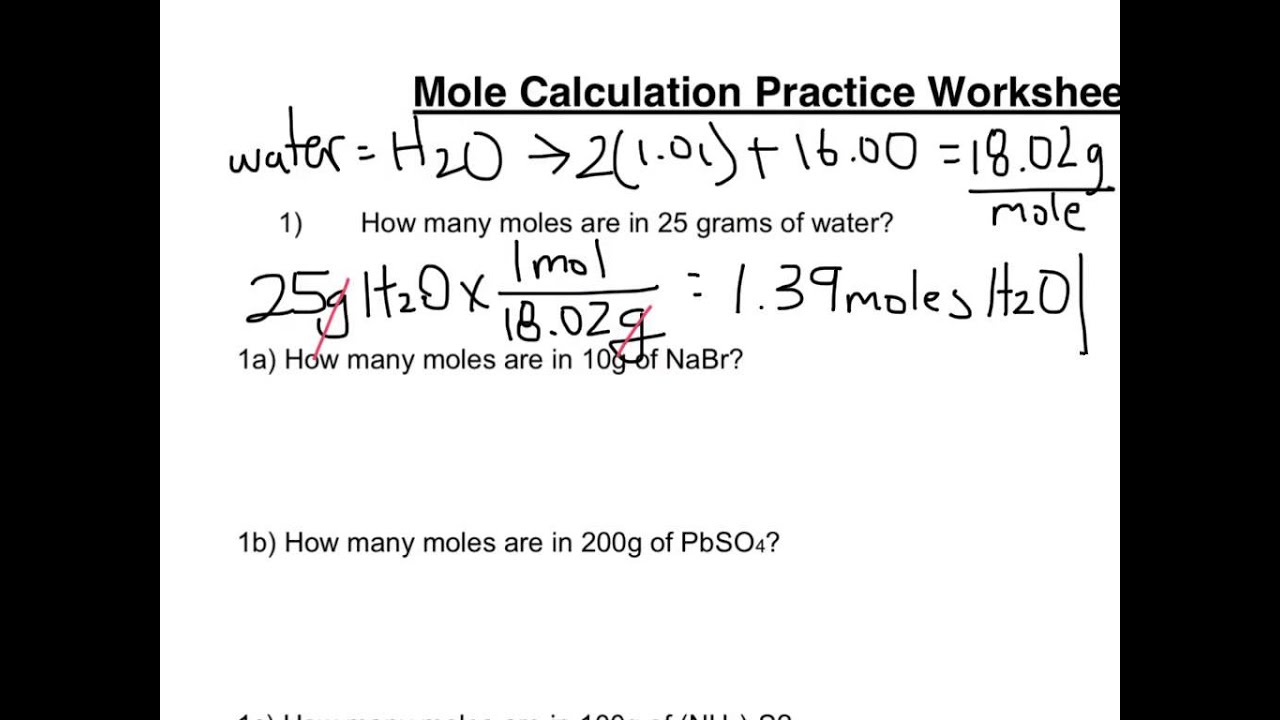
Mole worksheet #1
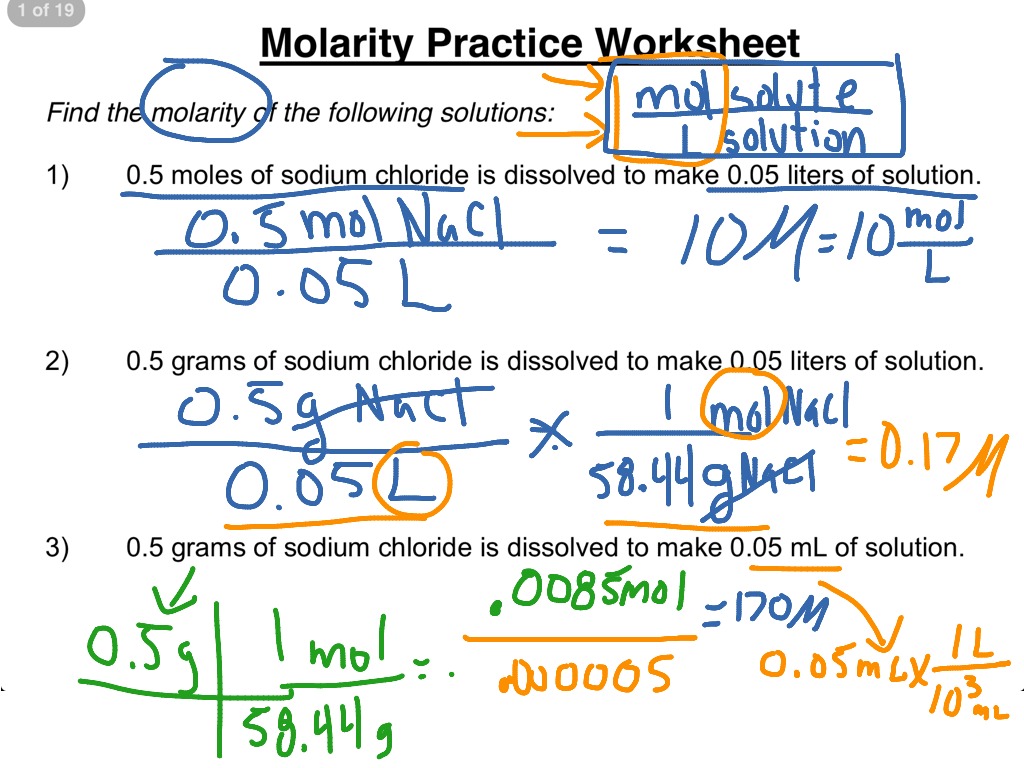
Molarity Worksheet # 1 106 g 1 mole Na 2 CO 3 1.09 moles 1 L . 3. Calculate the number of grams barium carbonate that can be precipitated by adding 50.0 mL of 0.424 MBa (NO 3) 2. Ba (NO 3) 2(aq) + K 2 CrO 4(aq) → BaCrO 4(s) + 2KNO 3(aq) 0.0500 L Ba (NO) 2 x 0.424 mole x 1 mole BaCO 3 x 253.3 g = 5.37 g BaCrO 4 A Mole of Moles - xkcd A Mole of Moles. What would happen if you were to gather a mole (unit of measurement) of moles (the small furry critter) in one place? —Sean Rice. Things get a bit gruesome. "One mole" is close to the number of atoms in a gram of hydrogen. It’s also, by chance, a decent ballpark guess for the number of grains of sand on Earth. Mole Calculation Worksheet - Everett Community College 1 mole Cd 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4? 4.3 x 1022 molecules H 3 PO 4 x 1 mole H 3 PO 4 = 7.1 x 10-2 moles H 3 PO 4 6.022 x 1023 molecules H 3 PO 4 5) How many molecules are in 48.0 grams of NaOH? 48.0 molecules NaOH x 1 mole NaOH x 6.022 x 1023 molecules NaOH 40 g NaOH 1 mole NaOH = 7.23 x 1023 molecules NaOH
Mole worksheet #1. Concentration Worksheet W 328 - Everett Community College O 1 mole Mg(CN) 2 4. 1.25 molal NaOH = 1.25 mole NaOH 1 kg H 2 O 1.25 mole NaOH x 40 g NaOH = 50.0 g NaOH 1 mole NaOH Measure 50.0 g NaOH and add water to 1 L volume. 5. 52 g K 2 SO 4 x 1 mole K 2 SO 4 = 0.299 mole K 2 SO 4 174 g K 2 SO 4 0.299 mole K 2 SO 4 = 0.073 M 4.100 L K 2 SO 4 6. 375 mL x 0.0750 = 28.125 mL ethylene glycol 28.125 mL ... Mole Calculation Worksheet - Everett Community College 1 mole Cd 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4? 4.3 x 1022 molecules H 3 PO 4 x 1 mole H 3 PO 4 = 7.1 x 10-2 moles H 3 PO 4 6.022 x 1023 molecules H 3 PO 4 5) How many molecules are in 48.0 grams of NaOH? 48.0 molecules NaOH x 1 mole NaOH x 6.022 x 1023 molecules NaOH 40 g NaOH 1 mole NaOH = 7.23 x 1023 molecules NaOH A Mole of Moles - xkcd A Mole of Moles. What would happen if you were to gather a mole (unit of measurement) of moles (the small furry critter) in one place? —Sean Rice. Things get a bit gruesome. "One mole" is close to the number of atoms in a gram of hydrogen. It’s also, by chance, a decent ballpark guess for the number of grains of sand on Earth. Molarity Worksheet # 1 106 g 1 mole Na 2 CO 3 1.09 moles 1 L . 3. Calculate the number of grams barium carbonate that can be precipitated by adding 50.0 mL of 0.424 MBa (NO 3) 2. Ba (NO 3) 2(aq) + K 2 CrO 4(aq) → BaCrO 4(s) + 2KNO 3(aq) 0.0500 L Ba (NO) 2 x 0.424 mole x 1 mole BaCO 3 x 253.3 g = 5.37 g BaCrO 4
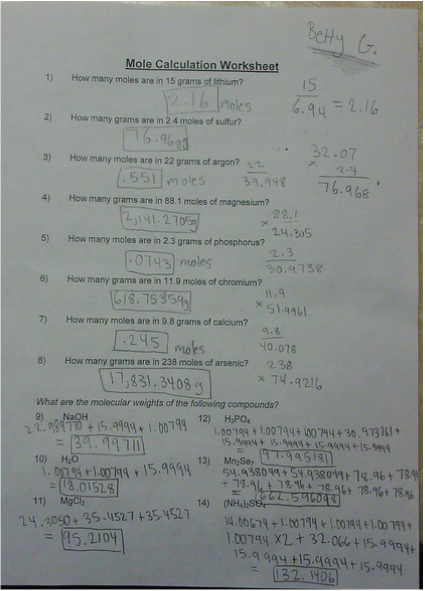




























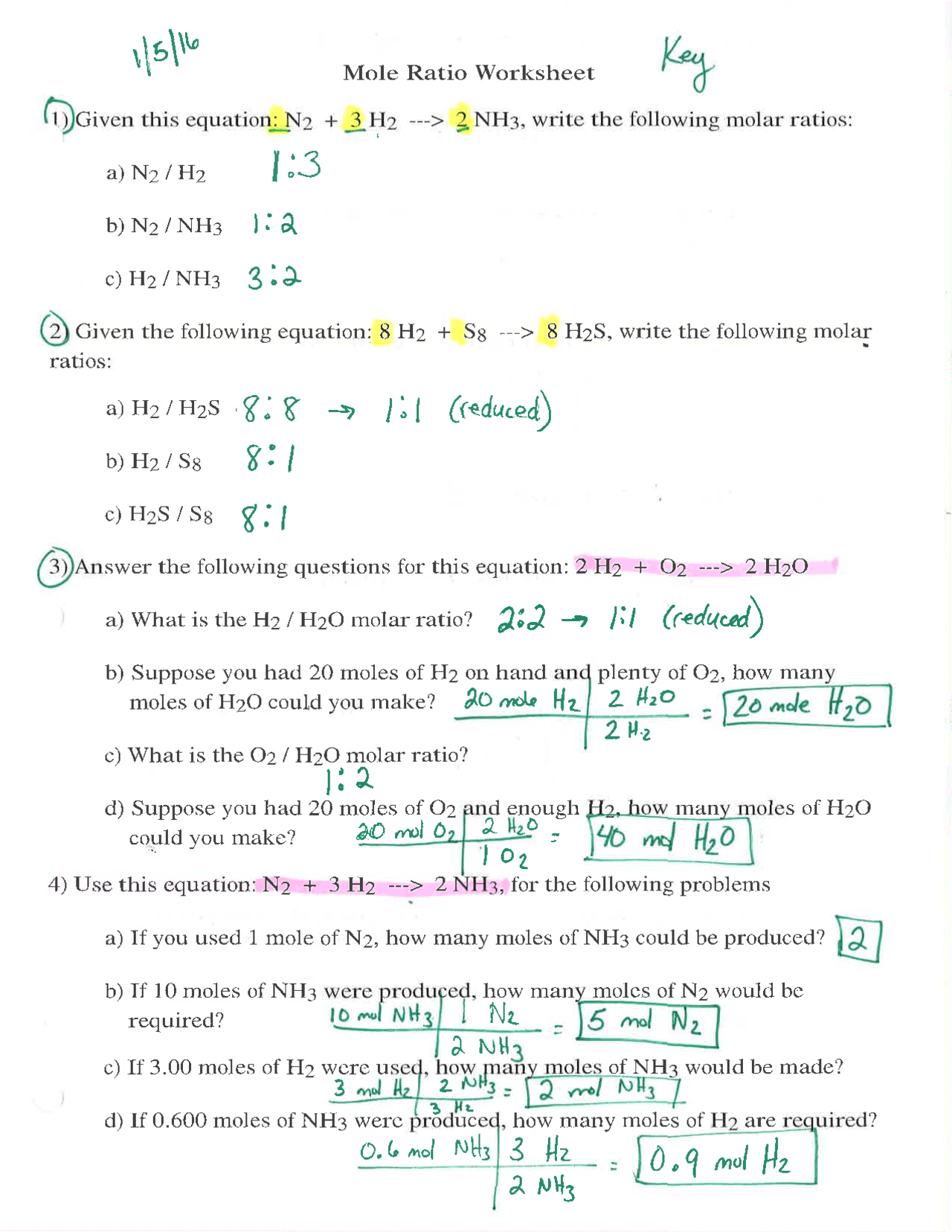

0 Response to "42 mole worksheet #1"
Post a Comment