40 mole ratio worksheet chemistry answers
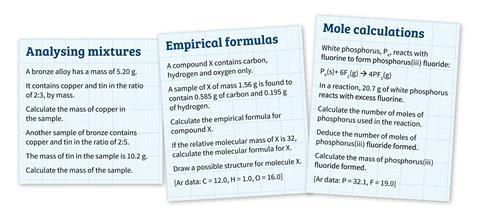
Calculating pH and pOH worksheet - Everett Community College Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of neutralization. There is more base than acid so the leftover base is what will affect the pH of the solution. 3.60 x 10-3 moles - 5.95 x 10-4 moles = 3.01 x 10-3 moles NaOH 3.01 x 10-3 moles NaOH = 3.01 x 10-3 M NaOH 1.00 L soln pOH = -log[OH-] = -log(3.01 x 10 ... How to Calculate the Empirical Formula of a Compound - dummies 21.07.2021 · An empirical formula represents the lowest whole-number ratio of elements in a compound. Here's how to find an empirical formula when given percent composition: Assume that you have 100 g of the unknown compound. The beauty of this little trick is that you conveniently gift yourself with the same number of grams of each elemental component as its contribution to …
Cambridge IGCSE Chemistry Coursebook 4th Edition Contents iii 10 Organic chemistry 252 Answers to questions 326 10.1 The unique properties of carbon 253 10.2 Alkanes 254 Glossary 336 10.3 Alkenes 257 Appendix: The Periodic Table 346 10.4 Hydrocarbon structure and isomerism 259 10.5 Chemical reactions of the alkanes 262 Index 347 10.6 Chemical reactions of the alkenes 263 Acknowledgements 353 ...

Mole ratio worksheet chemistry answers
Newton's Law of Universal Gravitation - Physics Classroom The solution of the problem involves substituting known values of G (6.673 x 10-11 N m 2 /kg 2), m 1 (5.98 x 10 24 kg), m 2 (70 kg) and d (6.39 x 10 6 m) into the universal gravitation equation and solving for F grav.The solution is as follows: Two general conceptual comments can be made about the results of the two sample calculations above. Cathode Ray Experiment by JJ.Thomson (CRT) - Explanation For better results in a cathode tube experiment, an evacuated (low pressure) tube is filled with hydrogen gas that is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio (e / m ratio = 1.76 x 10 ^ 11 coulombs per kg). Class 10 Chemistry Worksheets for CBSE NCERT (with Answers … We hope that our comprehensive post about CBSE Class 10 Chemistry Worksheet has given you all of the important information. As a result, students studying for tests must have excellent problem-solving abilities. To develop these abilities, students must complete enough Class 10 Chemistry revision worksheets.
Mole ratio worksheet chemistry answers. Mole Concept- Formula, Explanations, Examples, Related ... Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of ‘elementary entities’ of the given substance. The Avogadro number is represented by NA. The Mole Concept is a Convenient Method of Expressing the Amount of a Substance. To Learn more about the Mole Concept with Formulae and Examples with Videos and FAQs, The number of electrons in a ... NCERT Solutions For Class 12 Chemistry chapter 2-Solutions (i) Mole fraction (ii) Molality (iii) Molarity (iv) Mass percentage. Solution : (i) Mole fraction: The mole fraction of a component in a mixture is defined as the ratio of the number of moles of the component to the total number of moles of all the components in the mixture. i.e., Mole fraction of a component . Mole fraction is denoted by ‘x’. Chemistry Worksheets and Handouts (PDF for Printing) 08.03.2021 · This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. Chemistry Worksheets. Label Parts of the Atom [Google Apps worksheet][worksheet PDF][worksheet PNG][answers PNG] Acid formulas ; Balancing equations Worksheet #1 Biology Questions and Answers Form 1 - Biology Quizzes, Trivia, … f) Pair of forceps- This is an apparatus used for picking up small crawling animals e.g. stinging insects.. g) Specimen bottles- These are bottles used for keeping collected specimen.They are of different sizes depending on the size of the specimen being studied. h) Magnifying lens- This is used to enlarge small objects.A hand lens is a common magnifying lens used in the laboratory.
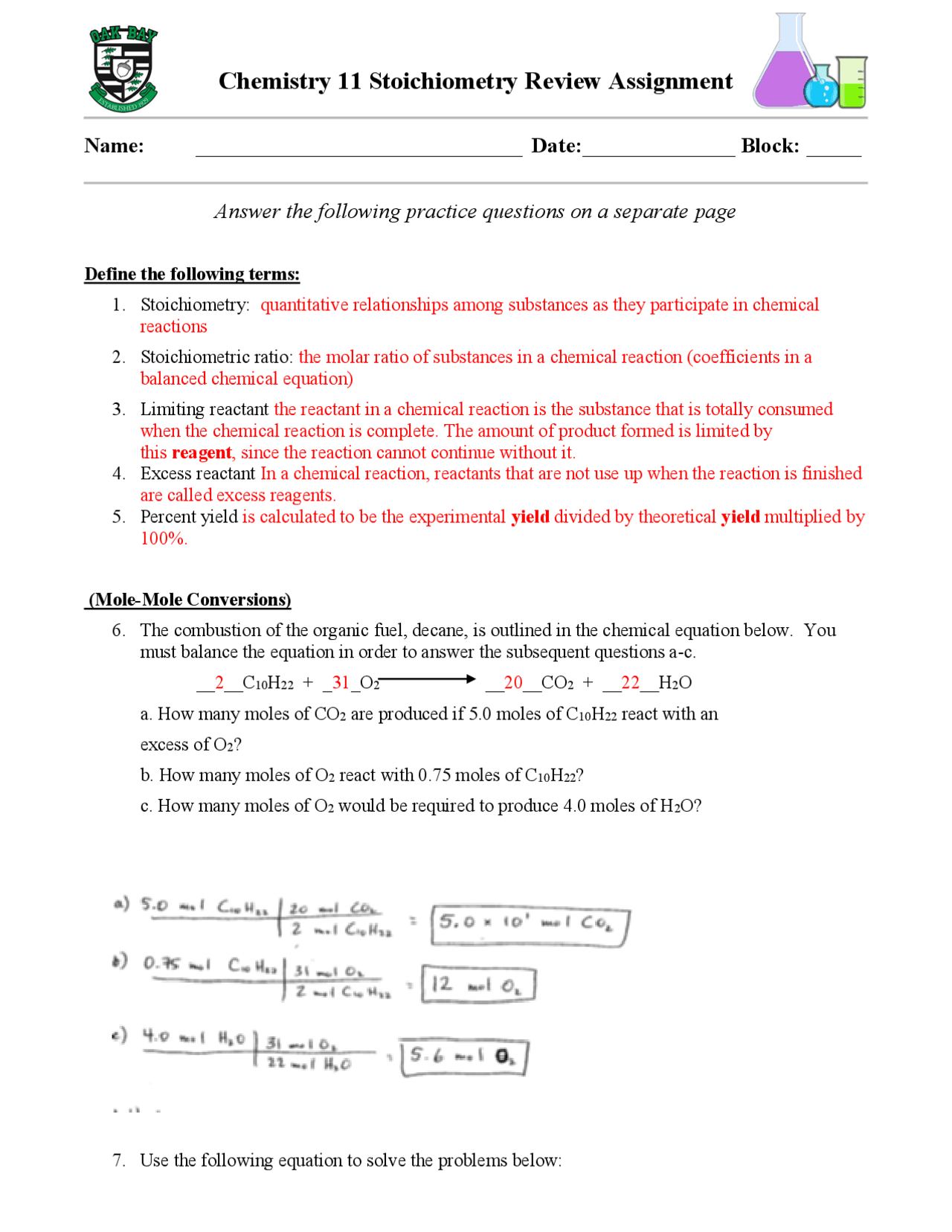
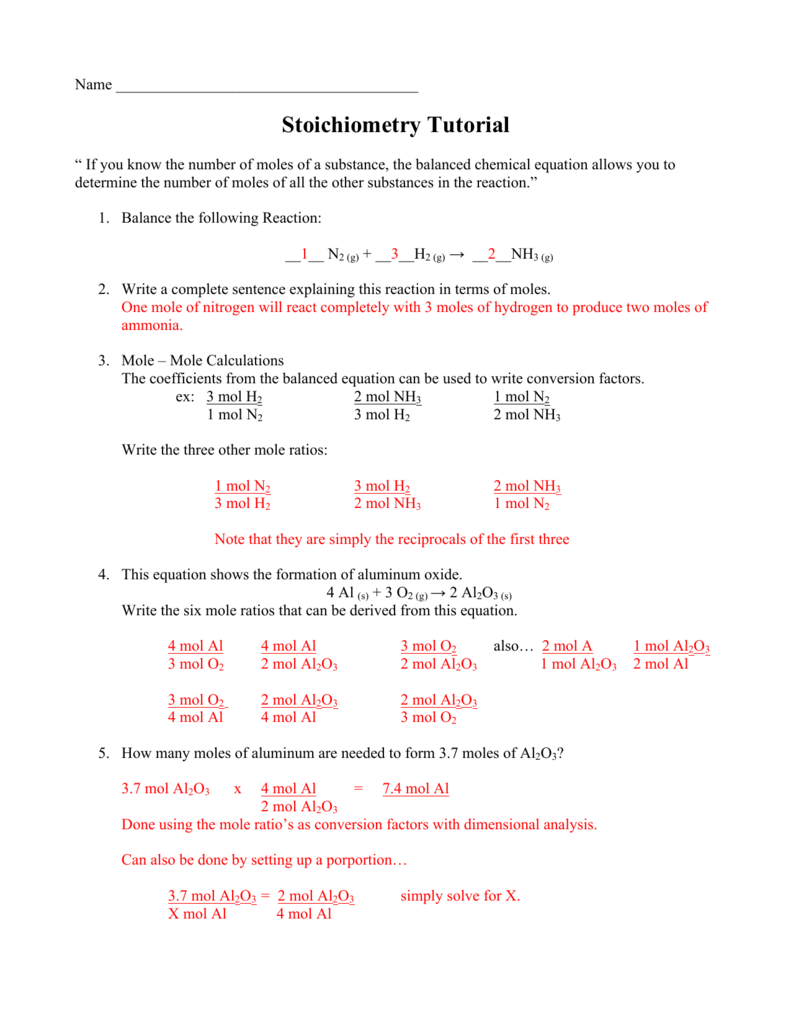
CHEMISTRY COMPUTING FORMULA MASS WORKSHEET - ISD 622 X = 90 g O2 1 mole O2 2 mole KClO3 122.5 g KClO3 = 229.7 g KClO3 32 g O2 3 moles O2 1 mole KClO3 I II III IV CHEMISTRY STOICHIOMETRY Mass (g,kg, etc.) Volume (L, mL. etc.) or # of Items (atoms,molec.) of Given reactant or product Moles of Given Mole Ratio from the Balanced Chemical Equation Overwatch 2 reaches 25 million players, tripling Overwatch 1 ... Oct 14, 2022 · Following a bumpy launch week that saw frequent server trouble and bloated player queues, Blizzard has announced that over 25 million Overwatch 2 players have logged on in its first 10 days."Sinc Cambridge IGCSE Chemistry Coursebook (fourth edition) - Issuu 09.06.2014 · Preview Cambridge IGCSE Chemistry Coursebook (fourth edition), Richard Harwood and Ian Lodge, Cambridge University Press. Class 12 Chemistry Worksheets for CBSE NCERT (with Answers … We hope that our comprehensive post about CBSE Class 12 Chemistry Worksheet has supplied you with all of the important information. As a result, students studying for tests must have excellent problem-solving abilities. To develop these abilities, students must complete enough Class 12 Chemistry revision worksheets.
Class 10 Chemistry Worksheets for CBSE NCERT (with Answers … We hope that our comprehensive post about CBSE Class 10 Chemistry Worksheet has given you all of the important information. As a result, students studying for tests must have excellent problem-solving abilities. To develop these abilities, students must complete enough Class 10 Chemistry revision worksheets. Cathode Ray Experiment by JJ.Thomson (CRT) - Explanation For better results in a cathode tube experiment, an evacuated (low pressure) tube is filled with hydrogen gas that is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio (e / m ratio = 1.76 x 10 ^ 11 coulombs per kg). Newton's Law of Universal Gravitation - Physics Classroom The solution of the problem involves substituting known values of G (6.673 x 10-11 N m 2 /kg 2), m 1 (5.98 x 10 24 kg), m 2 (70 kg) and d (6.39 x 10 6 m) into the universal gravitation equation and solving for F grav.The solution is as follows: Two general conceptual comments can be made about the results of the two sample calculations above.



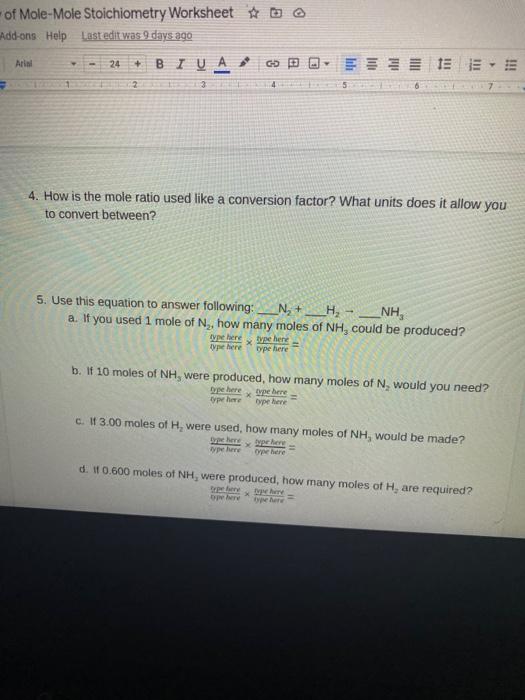
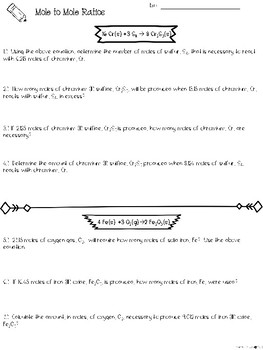









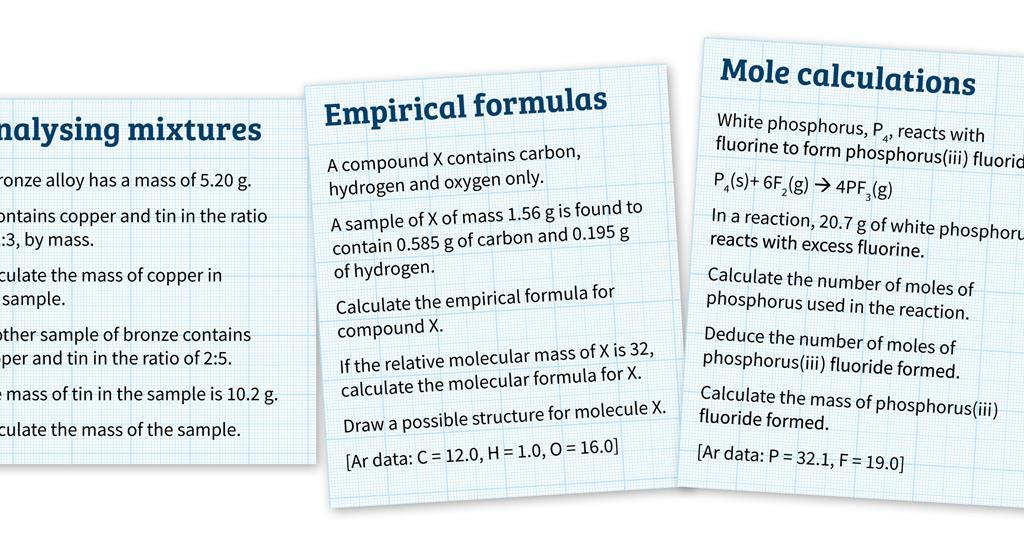

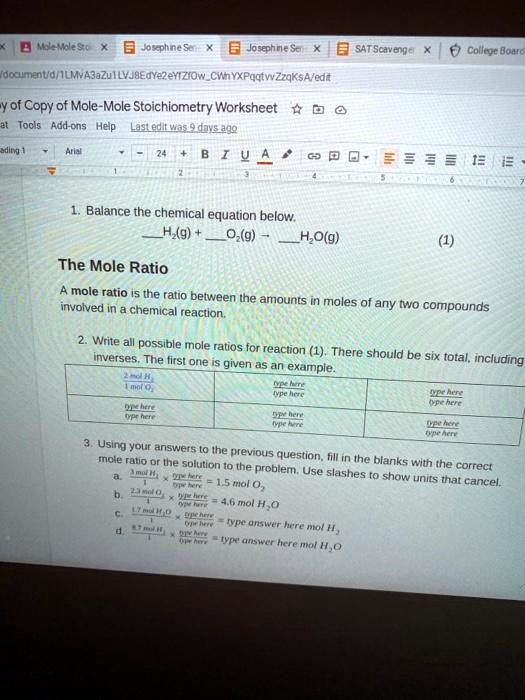












0 Response to "40 mole ratio worksheet chemistry answers"
Post a Comment